Potent and selective inhibition of SH3 domains with dirhodium metalloinhibitors
- PMID: 29142714
- PMCID: PMC5667506
- DOI: 10.1039/c5sc01602a
Potent and selective inhibition of SH3 domains with dirhodium metalloinhibitors
Abstract
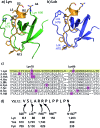
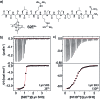
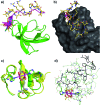
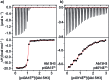
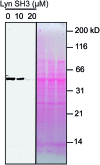
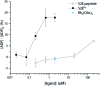
Src-family kinases (SFKs) play important roles in human biology and are key drug targets as well. However, achieving selective inhibition of individual Src-family kinases is challenging due to the high similarity within the protein family. We describe rhodium(ii) conjugates that deliver both potent and selective inhibition of Src-family SH3 domains. Rhodium(ii) conjugates offer dramatic affinity enhancements due to interactions with specific and unique Lewis-basic histidine residues near the SH3 binding interface, allowing predictable, structure-guided inhibition of SH3 targets that are recalcitrant to traditional inhibitors. In one example, a simple metallopeptide binds the Lyn SH3 domain with 6 nM affinity and exhibits functional activation of Lyn kinase under biologically relevant concentrations (EC50 ∼ 200 nM).
Figures
References
-
- Schreiner S. J., Schiavone A. P., Smithgall T. E. J. Biol. Chem. 2002;277:45680–45687. - PubMed
-
- Ingley E., Sarna M. K., Beaumont J. G., Tilbrook P. A., Tsai S., Takemoto Y., Williams J. H., Klinken S. P. J. Biol. Chem. 2000;275:7887–7893. - PubMed
-
- Takemoto Y., Sato M., Furuta M., Hashimoto Y. Int. Immunol. 1996;8:1699–1705. - PubMed
-
- Moarefi I., LaFevre-Bernt M., Sicheri F., Huse M., Lee C.-H., Kuriyan J., Miller W. T. Nature. 1997;385:650–653. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

