Access to enantioenriched 2,3- and 2,5-dihydrofurans with a fully substituted C2 stereocenter by Pd-catalyzed asymmetric intermolecular Heck reaction
- PMID: 29142715
- PMCID: PMC5667573
- DOI: 10.1039/c5sc01460c
Access to enantioenriched 2,3- and 2,5-dihydrofurans with a fully substituted C2 stereocenter by Pd-catalyzed asymmetric intermolecular Heck reaction
Abstract
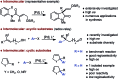
A palladium-catalyzed intermolecular asymmetric Heck reaction with dihydrofurans with a trisubstituted double bond is reported. The use of two different chiral ligands provides access to valuable 2,3- and 2,5-dihydrofurans with a fully substituted C2 stereocenter with high levels of regio- and enantiocontrol.
Figures
Similar articles
-
Decarboxylation-promoted Pd-catalyzed asymmetric propargylic [3 + 2] annulation for the enantioselective construction of a quaternary stereocenter in 2,3-dihydrofurans.Org Biomol Chem. 2018 Jan 31;16(5):742-749. doi: 10.1039/c7ob02778h. Org Biomol Chem. 2018. PMID: 29308470
-
Palladium-catalyzed asymmetric intermolecular Mizoroki-Heck reaction for construction of a chiral quaternary carbon center.Chem Commun (Camb). 2015 Aug 7;51(61):12235-8. doi: 10.1039/c5cc03601a. Chem Commun (Camb). 2015. PMID: 26136090
-
Sulfoxides as stereochemical controllers in intermolecular Heck reactions.Chemistry. 2001 Sep 17;7(18):3890-900. doi: 10.1002/1521-3765(20010917)7:18<3890::aid-chem3890>3.0.co;2-y. Chemistry. 2001. PMID: 11596931
-
Palladium/TY-Phos-Catalyzed Asymmetric Heck/Tsuji-Trost Reaction of o-Bromophenols with 1,3-Dienes.J Am Chem Soc. 2023 Mar 1;145(8):4378-4383. doi: 10.1021/jacs.2c12752. Epub 2023 Feb 16. J Am Chem Soc. 2023. PMID: 36795796
-
Pd-Catalyzed Asymmetric Dearomatization of Indoles via Decarbonylative Heck-Type Reaction of Thioesters.Org Lett. 2021 Jan 1;23(1):172-177. doi: 10.1021/acs.orglett.0c03897. Epub 2020 Dec 18. Org Lett. 2021. PMID: 33339458
Cited by
-
Asymmetric copper-catalyzed hydrophosphinylation of ethynylazaarenes to access P-chiral 2-azaaryl-ethylphosphine oxides.Chem Sci. 2025 Mar 3;16(14):5957-5966. doi: 10.1039/d5sc00358j. eCollection 2025 Apr 2. Chem Sci. 2025. PMID: 40060098 Free PMC article.
-
Assisted Tandem Pd Catalysis Enables Regiodivergent Heck Arylation of Transiently Generated Substituted Enol Ethers.JACS Au. 2023 Jan 12;3(1):261-274. doi: 10.1021/jacsau.2c00645. eCollection 2023 Jan 23. JACS Au. 2023. PMID: 36711081 Free PMC article.
References
-
- Carpenter N. E., Kucera D. J., Overman L. E. J. Org. Chem. 1989;54:5846.
- Sato Y., Sodeoka M., Shibasaki M. J. Org. Chem. 1989;54:4738.
-
- Oestreich M., The Mizoroki–Heck Reaction, Wiley, New York, 2009.
- Dounay A. B., Overman L. E. Chem. Rev. 2003;103:2945. - PubMed
- Shibasaki M., Vogl E. M., Ohshima T. Adv. Synth. Catal. 2004;346:1533.
- Tietze L. F., Ila H., Bell H. P. Chem. Rev. 2004;104:3453. - PubMed
- Diéguez M., Pàmies O. Isr. J. Chem. 2012;52:572.
- Oestreich M. Angew. Chem., Int. Ed. 2014;53:2282. - PubMed
-
- For a specific review on the intermolecular Heck reaction, see: Mc Cartney D., Guiry P. J., Chem. Soc. Rev., 2011, 40 , 5122 . - PubMed
-
- Ozawa F., Kubo A., Hayashi T. J. Am. Chem. Soc. 1991;113:1417.
- Ozawa F., Hayashi T. J. Organomet. Chem. 1992;428:267.
-
-
For selected leading examples, see:
- Ozawa F., Kobatake Y., Hayashi T. Tetrahedron Lett. 1993;34:2505.
- Kurihara Y., Sodeoka M., Shibasaki M. Chem. Pharm. Bull. 1994;42:2357.
- Loiseleur O., Hayashi M., Schmees N., Pfaltz A. Synthesis. 1997:1338.
- Hennessy A. J., Connolly D. J., Malone Y. M., Guiry P. J. Tetrahedron Lett. 2000;41:7757.
- Henriksen S. T., Norrby P.-O., Kaukoranta P., Andersson P. G. J. Am. Chem. Soc. 2008;130:10414. - PubMed
- Wöste T. H., Oestreich M. Chem.–Eur. J. 2011;17:11914. - PubMed
- Hu J., Hirao H., Li Y., Zhou J. Angew. Chem., Int. Ed. 2013;52:8676. - PubMed
- Liu S., Zhou J. Chem. Commun. 2013;49:11758. - PubMed
- Wu C., Zhou J. J. Am. Chem. Soc. 2014;136:650. - PubMed
-
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous