Fluorescence spectroscopy and microscopy as tools for monitoring redox transformations of uranium in biological systems
- PMID: 29142731
- PMCID: PMC5666681
- DOI: 10.1039/c5sc00661a
Fluorescence spectroscopy and microscopy as tools for monitoring redox transformations of uranium in biological systems
Abstract
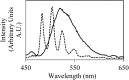
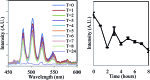
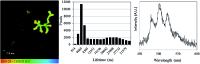
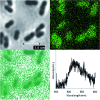
We report a study of redox reactions of uranium in model conditions using luminescence spectroscopy, which with its ease and wide availability has the potential to offer new insights into a bioremediation strategy of particular interest - the enzymatic reduction of UVIO22+ by bacteria such as Geobacter sulfurreducens. The inherent luminescent properties of UVIO22+ have been combined with confocal fluorescence microscopy techniques and lifetime image mapping to report directly on uranium concentration, localisation and oxidation state in cellular systems during uranium bioreduction, suggesting that localisation of uranyl species on the cell membrane surface plays an important role and that extracellular biogenic features form alongside uranyl sorbed cellular species during early stages of the bioreduction. The use of confocal microscopy in tandem with lifetime image mapping offers both improved temporal and spatial resolution (nanoseconds to microseconds and sub-micron respectively) than more conventional X-ray based techniques and offers the potential to image redox reactions occurring in situ. Together, these techniques provide an excellent and sensitive probe to assess the coordination environment of uranium during bioreduction processes that are currently being considered for remediation strategies of redox active radionuclides present in contaminated land.
Figures
Similar articles
-
Bioreduction of uranium: environmental implications of a pentavalent intermediate.Environ Sci Technol. 2005 Aug 1;39(15):5657-60. doi: 10.1021/es048232b. Environ Sci Technol. 2005. PMID: 16124300
-
Bioreduction of hydrogen uranyl phosphate: mechanisms and U(IV) products.Environ Sci Technol. 2013 Jun 4;47(11):5668-78. doi: 10.1021/es305258p. Epub 2013 May 21. Environ Sci Technol. 2013. PMID: 23634690
-
Modeling the inhibition of the bacteral reduction of U(VI) by beta-MnO2(s).Environ Sci Technol. 2002 Apr 1;36(7):1452-9. doi: 10.1021/es011159u. Environ Sci Technol. 2002. PMID: 11999050
-
Engineering and kinetic aspects of bacterial uranium reduction for the remediation of uranium contaminated environments.J Hazard Mater. 2019 Jun 5;371:198-212. doi: 10.1016/j.jhazmat.2019.02.074. Epub 2019 Feb 21. J Hazard Mater. 2019. PMID: 30851673 Review.
-
Uranium speciation and bioavailability in aquatic systems: an overview.ScientificWorldJournal. 2002 Mar 15;2:707-29. doi: 10.1100/tsw.2002.130. ScientificWorldJournal. 2002. PMID: 12805996 Free PMC article. Review.
Cited by
-
Comparative analysis of uranium bioassociation with halophilic bacteria and archaea.PLoS One. 2018 Jan 12;13(1):e0190953. doi: 10.1371/journal.pone.0190953. eCollection 2018. PLoS One. 2018. PMID: 29329319 Free PMC article.
-
Inducing fluorescence of uranyl acetate as a dual-purpose contrast agent for correlative light-electron microscopy with nanometre precision.Sci Rep. 2017 Sep 5;7(1):10442. doi: 10.1038/s41598-017-10905-x. Sci Rep. 2017. PMID: 28874723 Free PMC article.
-
Correlative fluorescence microscopy, transmission electron microscopy and secondary ion mass spectrometry (CLEM-SIMS) for cellular imaging.PLoS One. 2021 May 10;16(5):e0240768. doi: 10.1371/journal.pone.0240768. eCollection 2021. PLoS One. 2021. PMID: 33970908 Free PMC article.
-
Sensing Uranyl(VI) Ions by Coordination and Energy Transfer to a Luminescent Europium(III) Complex.Angew Chem Int Ed Engl. 2018 Jul 26;57(31):9921-9924. doi: 10.1002/anie.201805316. Epub 2018 Jun 29. Angew Chem Int Ed Engl. 2018. PMID: 29898241 Free PMC article.
-
Biological Reduction of a U(V)-Organic Ligand Complex.Environ Sci Technol. 2021 Apr 20;55(8):4753-4761. doi: 10.1021/acs.est.0c06633. Epub 2021 Mar 11. Environ Sci Technol. 2021. PMID: 33705103 Free PMC article.
References
-
- Valko M., Morris H., Cronin M. T. D. Curr. Med. Chem. 2005;12:1161–1208. - PubMed
-
- Pawlicki M., Collins H. A., Denning R. G., Anderson H. L. Angew. Chem., Int. Ed. 2009;48:3244–3266. - PubMed
-
- Hell S. W., Wichmann J. Opt. Lett. 1994;19:780–782. - PubMed
-
- Konig K. J. Microsc. 2000;200:83–104. - PubMed
-
- Sarkar A. R., Kang D. E., Kim H. M., Cho B. R. Inorg. Chem. 2014;53:1794–1803. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

