Enantioselective palladium(0)-catalyzed intramolecular cyclopropane functionalization: access to dihydroquinolones, dihydroisoquinolones and the BMS-791325 ring system
- PMID: 29142735
- PMCID: PMC5667185
- DOI: 10.1039/c5sc01909e
Enantioselective palladium(0)-catalyzed intramolecular cyclopropane functionalization: access to dihydroquinolones, dihydroisoquinolones and the BMS-791325 ring system
Abstract
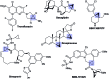
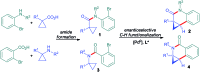
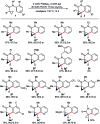
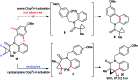
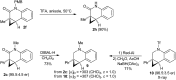
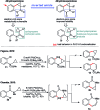
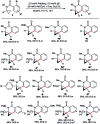
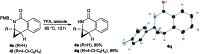
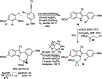
Taddol-based phosphoramidite ligands enable enantioselective palladium(0)-catalyzed C-H arylation of cyclopropanes. The cyclized products are obtained in high yields and enantioselectivities. The reported method provides efficient access to a broad range of synthetically attractive cyclopropyl containing dihydroquinolones and dihydroisoquinolones as well as allows for an efficient enantioselective construction of the 7-membered ring of the cyclopropyl indolobenzazepine core of BMS-791325.
Figures
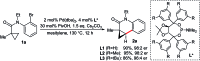


References
-
- Gootz T. D., Zaniewski R., Haskell S., Schmieder B., Tankovic J., Girard D., Courvalin P., Polzer R. J. Antimicrob. Agents Chemother. 2006;40:2691. - PMC - PubMed
- Augeri D. J., Robl J. A., Betebenner D. A., Magnin D. R., Khanna A., Robertson J. G., Wang A., Simpkins L. M., Taunk P., Huang Q., Han S.-P., Abboa-Offei B., Cap M., Xin L., Tao L., Tozzo E., Welzel G. E., Egan D. M., Marcinkeviciene J., Chang S. Y., Biller S. A., Kirby M. S., Parker R. A., Hamann L. G. J. Med. Chem. 2005;48:5025. - PubMed
- Micheli F., Cavanni P., Andreotti D., Arban R., Benedetti R., Bertani B., Bettati M., Bettelini L., Bonanomi G., Braggio S., Carletti R., Checchia A., Corsi M., Fazzolari E., Fontana S., Marchioro C., Merlo-Pich E., Negri M., Oliosi B., Ratti E., Read K. D., Roscic M., Sartori I., Spada S., Tedesco G., Tarsi L., Terreni S., Visentini F., Zocchi A., Zonzini L. J. Med. Chem. 2000;53:4989. - PubMed
- Krattenmacher R. Contraception. 2000;62:29. - PubMed
- Lin T. I., Lenz O., Fanning G., Verbinnen T., Delouvroy F., Scholliers A., Vermeiren K., Rosenquist A., Edlund M., Samuelsson B., Vrang L., De Kock H., Wigerinck P., Raboisson P., Simmen K. Antimicrob. Agents Chemother. 2009;53:1377. - PMC - PubMed
- Gentles R. G., Ding M., Bender J. A., Bergstrom C. P., Grant-Young K., Hewawasam P., Hudyma T., Martin S., Nickel A., Regueiro-Ren A., Tu Y., Yang Z., Yeung K.-S., Zheng X., Chao S., Sun J.-H., Beno B. R., Camac D. M., Chang C.-H., Gao M., Morin P. E., Sheriff S., Tredup J., Wan J., Witmer M. R., Xie D., Hanumegowda U., Knipe J., Mosure K., Santone K. S., Parker D. D., Zhuo X., Lemm J., Liu M., Pelosi L., Rigat K., Voss S., Wang Y., Wang Y.-K., Colonno R. J., Gao M., Roberts S. B., Gao Q., Ng A., Meanwell N. A., Kadow J. F. J. Med. Chem. 2014;57:1855. - PubMed
-
- Lebel H., Marcoux J.-F., Molinaro C., Charette A. B. Chem. Rev. 2003;103:977. - PubMed
- Pellissier H. Tetrahedron. 2008;64:7041.
-
- Eaton P. E., Lee C.-H., Xiong Y. J. Am. Chem. Soc. 1989;111:8016.
- Zhang M.-X., Eaton P. E. Angew. Chem., Int. Ed. 2002;41:2169. - PubMed
-
- Lauru S., Simpkins N. S., Gethin D., Wilson C. Chem. Commun. 2008:5390. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

