Intraductal papillary mucinous neoplasm of the pancreas rapidly xenografts in chicken eggs and predicts aggressiveness
- PMID: 29143337
- PMCID: PMC5836935
- DOI: 10.1002/ijc.31160
Intraductal papillary mucinous neoplasm of the pancreas rapidly xenografts in chicken eggs and predicts aggressiveness
Abstract
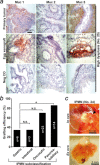
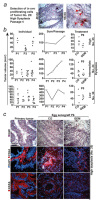
Intraductal papillary mucinous neoplasm (IPMN) of the pancreas has a high risk of progressing to invasive pancreatic ductal adenocarcinoma (PDA), but experimental models for IPMN are largely missing. New experimental systems for the molecular characterization of IPMN and for personalized prognosis and treatment options for IPMN are urgently needed. We analyzed the potential use of fertilized chicken eggs for the culture of freshly resected IPMN tissue. We transplanted 49 freshly resected IPMN tissues into eggs and compared the growth characteristics to IPMN tissues transplanted into mice; this was followed by an analysis of histology, morphology, and marker expression. Of the IPMN tissues transplanted into eggs, 63% formed tumor xenografts within 4 days, while none of the 12 IPMN tissues transplanted into immunodeficient mice engrafted. In the eggs, the grafting efficiency of high-grade (n = 14) and intermediate-grade (n = 17) dysplasia was 77% and was significantly higher than the 39% grafting efficiency of low-grade dysplasia (n = 18). According to mucinous expression, 46 IPMN tissues were classified into gastric (n = 6), intestinal (n = 3), oncocytic (n = 23), and pancreatobiliary (n = 14) subtypes. The grafting efficiency was highest for the pancreatobiliary subtype (86%), followed by the oncocytic (70%), gastric (33%) and intestinal (33%) subtypes. The morphology and expression patterns of mucins, progression markers and pancreatic ductal markers were comparable between the primary IPMN tissues and their xenograft copies. The individual tumor environment was largely maintained during subtransplantation, as evaluated upon passage 6. This new IPMN model may facilitate experimental studies and treatment decisions for the optimal personalized management of IPMN.
Keywords: IPMN of the pancreas; chorioallantoic membrane; xenograft models.
© 2017 The Authors International Journal of Cancer published by John Wiley & Sons Ltd on behalf of UICC.
Figures



References
-
- Ohashi KMY, Maruyama M, Takekoshi T, et al. Four cases of mucous‐secreting pancreatic cancer. Prog Dig Endosc 1982;20:348–51.
-
- Simons JP, Ng SC, Shah SA, et al. Malignant intraductal papillary mucinous neoplasm: are we doing the right thing?. J Surg Res 2011;167:251–7. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

