K2P TASK-2 and KCNQ1-KCNE3 K+ channels are major players contributing to intestinal anion and fluid secretion
- PMID: 29143340
- PMCID: PMC5792569
- DOI: 10.1113/JP275178
K2P TASK-2 and KCNQ1-KCNE3 K+ channels are major players contributing to intestinal anion and fluid secretion
Abstract
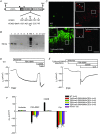
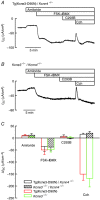
Key points: K+ channels are important in intestinal epithelium as they ensure the ionic homeostasis and electrical potential of epithelial cells during anion and fluid secretion. Intestinal epithelium cAMP-activated anion secretion depends on the activity of the (also cAMP dependent) KCNQ1-KCNE3 K+ channel, but the secretory process survives after genetic inactivation of the K+ channel in the mouse. Here we use double mutant mice to investigate which alternative K+ channels come into action to compensate for the absence of KCNQ1-KCNE3 K+ channels. Our data establish that whilst Ca2+ -activated KCa 3.1 channels are not involved, K2P two-pore domain TASK-2 K+ channels are major players providing an alternative conductance to sustain the intestinal secretory process. Work with double mutant mice lacking both TASK-2 and KCNQ1-KCNE3 channels nevertheless points to yet-unidentified K+ channels that contribute to the robustness of the cAMP-activated anion secretion process.
Abstract: Anion and fluid secretion across the intestinal epithelium, a process altered in cystic fibrosis and secretory diarrhoea, is mediated by cAMP-activated CFTR Cl- channels and requires the simultaneous activity of basolateral K+ channels to maintain cellular ionic homeostasis and membrane potential. This function is fulfilled by the cAMP-activated K+ channel formed by the association of pore-forming KCNQ1 with its obligatory KCNE3 β-subunit. Studies using mice show sizeable cAMP-activated intestinal anion secretion in the absence of either KCNQ1 or KCNE3 suggesting that an alternative K+ conductance must compensate for the loss of KCNQ1-KCNE3 activity. We used double mutant mouse and pharmacological approaches to identify such a conductance. Ca2+ -dependent anion secretion can also be supported by Ca2+ -dependent KCa 3.1 channels after independent CFTR activation, but cAMP-dependent anion secretion is not further decreased in the combined absence of KCa 3.1 and KCNQ1-KCNE3 K+ channel activity. We show that the K2P K+ channel TASK-2 is expressed in the epithelium of the small and large intestine. Tetrapentylammonium, a TASK-2 inhibitor, abolishes anion secretory current remaining in the absence of KCNQ1-KCNE3 activity. A double mutant mouse lacking both KCNQ1-KCNE3 and TASK-2 showed a much reduced cAMP-mediated anion secretion compared to that observed in the single KCNQ1-KCNE3 deficient mouse. We conclude that KCNQ1-KCNE3 and TASK-2 play major roles in the intestinal anion and fluid secretory phenotype. The persistence of an, admittedly reduced, secretory activity in the absence of these two conductances suggests that further additional K+ channel(s) as yet unidentified contribute to the robustness of the intestinal anion secretory process.
Keywords: K+ channel; epithelial transport; fluid secretion.
© 2017 The Authors. The Journal of Physiology © 2017 The Physiological Society.
Figures




Comment in
-
Taking intestinal anion secretion to TASK: a role for K2P channels in cyclic AMP-regulated anion secretion.J Physiol. 2018 Feb 1;596(3):359-360. doi: 10.1113/JP275567. Epub 2017 Dec 27. J Physiol. 2018. PMID: 29205365 Free PMC article. No abstract available.
-
Enterocyte K+ ion permeability and fluid secretion: missing the correct channel or missing the point?J Physiol. 2018 Jun;596(12):2463-2464. doi: 10.1113/JP276102. Epub 2018 Apr 25. J Physiol. 2018. PMID: 29604065 Free PMC article. No abstract available.
-
Reply from L. P. Cid, T. J. Jentsch and F. V. Sepúlveda: intestinal electrolyte and fluid secretion - a model in trouble?J Physiol. 2018 Jun;596(12):2465-2466. doi: 10.1113/JP276139. Epub 2018 Apr 16. J Physiol. 2018. PMID: 29663391 Free PMC article. No abstract available.
References
-
- Abbott GW & Goldstein SA (2002). Disease‐associated mutations in KCNE potassium channel subunits (MiRPs) reveal promiscuous disruption of multiple currents and conservation of mechanism. FASEB J 16, 390–400. - PubMed
-
- Al‐Hazza A, Linley JE, Aziz Q, Maclennan KA, Hunter M & Sandle GI (2012). Potential role of reduced basolateral potassium (IKCa3.1) channel expression in the pathogenesis of diarrhoea in ulcerative colitis. J Pathol 226, 463–470. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

