Pharmacokinetics of concentrated naloxone nasal spray for opioid overdose reversal: Phase I healthy volunteer study
- PMID: 29143400
- PMCID: PMC5836974
- DOI: 10.1111/add.14033
Pharmacokinetics of concentrated naloxone nasal spray for opioid overdose reversal: Phase I healthy volunteer study
Abstract
Background and aims: Take-home naloxone can prevent death from heroin/opioid overdose, but pre-provision is difficult because naloxone is usually given by injection. Non-injectable alternatives, including naloxone nasal sprays, are currently being developed. To be effective, the intranasal (i.n.) spray dose must be adequate but not excessive, and early absorption must be comparable to intramuscular (i.m.) injection. We report on the pharmacokinetics (PK) of a specially produced concentrated novel nasal spray. The specific aims were to: (1) estimate PK profiles of i.n. naloxone, (2) compare early systemic exposure with i.n. versus i.m. naloxone and (3) estimate i.n. bioavailability.
Design: Open-label, randomized, five-way cross-over PK study.
Setting: Clinical trials facility (Croydon, UK).
Participants: Thirty-eight healthy volunteers (age 20-54 years; 11 female).
Intervention and comparator: Three doses of i.n. (1 mg/0.1 ml, 2 mg/0.1 ml, 4 mg/0.2 ml) versus 0.4 mg i.m. (reference) and 0.4 mg intravenous (i.v.) naloxone.
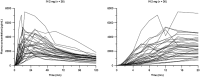
Measurements: Regular blood samples were taken, with high-frequency sampling during the first 15 minutes to capture early systemic exposure. PK parameters were determined from plasma naloxone concentrations. Exploratory analyses involved simulation of repeat administration.
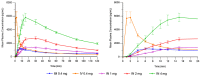
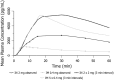
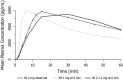
Findings: Mean peak concentration (Cmax ) values for 1 mg (1.51 ng/ml), 2 mg (2.87 ng/ml) and 4 mg (6.02 ng/ml) i.n. exceeded 0.4 mg i.m. (1.27 ng/ml) naloxone. All three i.n. doses rapidly achieved plasma levels > 50% of peak concentrations (T50%) by 10 minutes, peaking at 15-30 minutes (Tmax ). For comparison, the i.m. reference reached Tmax at 10 minutes. Mean bioavailability was 47-51% for i.n. relative to i.m. naloxone. Simulation of repeat dosing (2 × 2 mg i.n. versus 5 × 0.4 mg i.m. doses) at 3-minute intervals showed that comparable plasma naloxone concentrations would be anticipated.
Conclusions: Concentrated 2 mg intranasal naloxone is well-absorbed and provides early exposure comparable to 0.4 mg intramuscular naloxone, following the 0.4 mg intramuscular curve closely in the first 10 minutes post-dosing and maintaining blood levels above twice the intramuscular reference for the next 2 hours.
Keywords: Antidote; drug overdose; intranasal; naloxone; nasal; opiate; opioids; pharmacokinetics.
© 2017 The Authors. Addiction published by John Wiley & Sons Ltd on behalf of Society for the Study of Addiction.
Figures
Comment in
-
Commentary on McDonald et al. (2018): Intranasal naloxone-from the laboratory to the real world.Addiction. 2018 Mar;113(3):494-495. doi: 10.1111/add.14087. Addiction. 2018. PMID: 29423984 No abstract available.
References
-
- United Nations Office on Drugs and Crime (UNODC) . World Drug Report. 2016. Available at: https://www.unodc.org/wdr2016/ (accessed 1 December 2016) (Archived at http://www.webcitation.org/6u56T1R41 on 9 October 2017).
-
- World Health Organization (WHO) . Community Management of Opioid Overdose 2014. Available at: http://apps.who.int/iris/bitstream/10665/137462/1/9789241548816_eng.pdf?... (accessed 25 January 2016) (Archived at http://www.webcitation.org/6qHBtnGWj on 7 May 2017).
-
- Strang J. Take‐home naloxone: the next steps. Comments on Strang et al.'s ‘Preventing opiate overdose fatalities with take‐home naloxone: pre‐launch study of possible impact and acceptability’. Addiction 1999; 94: 206–207. - PubMed
-
- Strang J., McDonald R., Tas B., Day E. Clinical provision of improvised nasal naloxone without experimental testing and without regulatory approval: imaginative shortcut or dangerous bypass of essential safety procedures? Addiction 2016; 111: 574–582. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

