Safety and tolerability of inebilizumab (MEDI-551), an anti-CD19 monoclonal antibody, in patients with relapsing forms of multiple sclerosis: Results from a phase 1 randomised, placebo-controlled, escalating intravenous and subcutaneous dose study
- PMID: 29143550
- PMCID: PMC6360486
- DOI: 10.1177/1352458517740641
Safety and tolerability of inebilizumab (MEDI-551), an anti-CD19 monoclonal antibody, in patients with relapsing forms of multiple sclerosis: Results from a phase 1 randomised, placebo-controlled, escalating intravenous and subcutaneous dose study
Abstract
Background: B cells may be involved in the pathophysiology of multiple sclerosis (MS). Inebilizumab (formerly MEDI-551) binds to and depletes CD19+ B cells.
Objectives: To assess safety, tolerability, pharmacokinetics, pharmacodynamics and immunogenicity of inebilizumab in adults with relapsing MS.
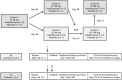
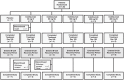
Methods: This phase 1 trial randomised 28 patients 3:1 (21, inebilizumab; 7, placebo) to inebilizumab (2 intravenous (IV) doses, days 1 and 15: 30, 100 or 600 mg; or single subcutaneous (SC) dose on day 1: 60 or 300 mg) or matching placebo, with follow-up until at least week 24 or return of CD19+ B-cell count to ⩾80 cells/µL.
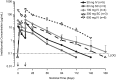
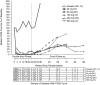
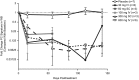
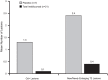
Results: Complete B-cell depletion was observed across all doses. Infusion/injection (grade 1/2) reactions occurred in 6/15 patients receiving inebilizumab IV, 2/5 placebo IV and 1/6 inebilizumab SC. Serious adverse events occurred in three patients receiving inebilizumab: pyrexia, mixed-drug intoxication (unrelated to inebilizumab; resulted in death) and urinary tract infection. Mean number of cumulative new gadolinium-enhancing lesions over 24 weeks was 0.1 with inebilizumab versus 1.3 with placebo; mean numbers of new/newly enlarging T2 lesions were 0.4 and 2.4, respectively.
Conclusion: Inebilizumab had an acceptable safety profile in relapsing MS patients and showed a trend in reductions in new/newly enlarging and gadolinium-enhancing lesions.
Keywords: B cells; intravenous administration; pharmacodynamics; pharmacokinetics; subcutaneous administration.
Conflict of interest statement
Figures
References
-
- Baranzini SE, Jeong MC, Butunoi C, et al. B cell repertoire diversity and clonal expansion in multiple sclerosis brain lesions. J Immunol 1999; 163: 5133–5144. - PubMed
-
- Duddy M, Niino M, Adatia F, et al. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol 2007; 178: 6092–6099. - PubMed
-
- Faissner S, Nikolayczik J, Chan A, et al. Plasmapheresis and immunoadsorption in patients with steroid refractory multiple sclerosis relapses. J Neurol 2016; 263: 1092–1098. - PubMed
-
- Knippenberg S, Peelen E, Smolders J, et al. Reduction in IL-10 producing B cells (Breg) in multiple sclerosis is accompanied by a reduced naive/memory Breg ratio during a relapse but not in remission. J Neuroimmunol 2011; 239: 80–86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

