Mammalian target of rapamycin complex 2 (mTORC2) controls glycolytic gene expression by regulating Histone H3 Lysine 56 acetylation
- PMID: 29143563
- PMCID: PMC5815439
- DOI: 10.1080/15384101.2017.1404207
Mammalian target of rapamycin complex 2 (mTORC2) controls glycolytic gene expression by regulating Histone H3 Lysine 56 acetylation
Abstract
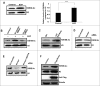
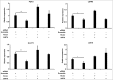
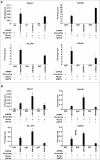
Metabolic reprogramming is a hallmark of cancer cells, but the mechanisms are not well understood. The mammalian target of rapamycin complex 2 (mTORC2) controls cell growth and proliferation and plays a critical role in metabolic reprogramming in glioma. mTORC2 regulates cellular processes such as cell survival, metabolism, and proliferation by phosphorylation of AGC kinases. Components of mTORC2 are shown to localize to the nucleus, but whether mTORC2 modulates epigenetic modifications to regulate gene expression is not known. Here, we identified histone H3 lysine 56 acetylation (H3K56Ac) is regulated by mTORC2 and show that global H3K56Ac levels were downregulated on mTORC2 knockdown but not on mTORC1 knockdown. mTORC2 promotes H3K56Ac in a tuberous sclerosis complex 1/2 (TSC1/2) mediated signaling pathway. We show that knockdown of sirtuin6 (SIRT6) prevented H3K56 deacetylation in mTORC2 depleted cells. Using glioma model consisting of U87EGFRvIII cells, we established that mTORC2 promotes H3K56Ac in glioma. Finally, we show that mTORC2 regulates the expression of glycolytic genes by regulating H3K56Ac levels at the promoters of these genes in glioma cells and depletion of mTOR leads to increased recruitment of SIRT6 to these promoters. Collectively, these results identify mTORC2 signaling pathway positively promotes H3K56Ac through which it may mediate metabolic reprogramming in glioma.
Keywords: Cancer cells; EGFR; Glioma; Histone acetylation; Histone deacetylases; SIRT6; TSC1/2; growth factors; mTOR; metabolic reprogramming; metabolism; signal transduction.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
