Survey of programmatic experiences and challenges in delivery of hepatitis B and C testing in low- and middle-income countries
- PMID: 29143609
- PMCID: PMC5688462
- DOI: 10.1186/s12879-017-2767-0
Survey of programmatic experiences and challenges in delivery of hepatitis B and C testing in low- and middle-income countries
Abstract
Background: There have been few reports on programmatic experience of viral hepatitis testing and treatment in resource-limited settings. To inform the development of the 2017 World Health Organization (WHO) viral hepatitis testing guidance and in particular the feasibility of proposed recommendations, we undertook a survey across a range of organisations engaged with hepatitis testing in low- and middle-income countries (LMICs). Our objective was to describe current hepatitis B and C testing practices across a range of settings in different countries, as well as key barriers or challenges encountered and proposed solutions to promote testing scale-up.
Methods: Hepatitis testing programmes in predominantly LMICs were identified from the WHO Global Hepatitis Programme contacts database and through WHO regional offices, and invited to participate. The survey comprised a six-part structured questionnaire: general programme information, description of hepatitis testing, treatment and care services, budget and funding, data on programme outcomes, and perceptions on key barriers encountered and strategies to address these.
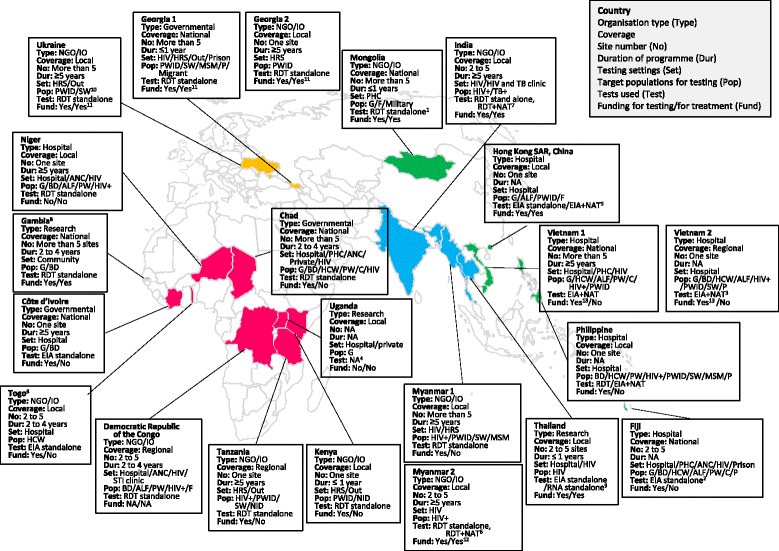
Results: We interviewed 22 viral hepatitis testing programmes from 19 different countries. Nine were from the African region; 6 from the Western Pacific; 4 from South-East Asia; and 3 from Eastern Europe. All but four of the programmes were based in LMICs, and 10 (45.5%) were supported by non-governmental or international organizations. All but two programmes undertook targeted testing of specific affected populations such as people living with HIV, people who inject drugs, sex workers, health care workers, and pregnant women. Only two programmes focussed on routine testing in the general population. The majority of programmes were testing in hospital-based or other health facilities, particularly HIV clinics, and community-based testing was limited. Nucleic acid testing (NAT) for confirmation of HCV and HBV viraemia was available in only 30% and 18% of programmes, respectively. Around a third of programmes required some patient co-payment for diagnosis. The most commonly identified challenges in scale-up of hepatitis testing were: limited community awareness about viral hepatitis; lack of facilities or services for hepatitis testing; no access to low cost treatment, particularly for HCV; absence of national guidance and policies; no dedicated budget for hepatitis; and lack of trained health care and laboratory workers.
Conclusions: At this early stage in the global scale-up of testing for viral hepatitis, there is a wide variation in testing practices and approaches across different programmes. There remains limited access to NAT to confirm viraemia, and patient self-payment for testing and treatment is common. There was consensus from implementing organizations that scale-up of testing will require increased community awareness, health care worker training, development of national strategies and guidelines, and improved access to low cost NAT virological testing.
Keywords: Feasibility; Hepatitis testing; Low- and middle-income countries; Programme experience; WHO guidelines on hepatitis B and C testing.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- World Health Organization. Global Hepatitis Report 2017. 2017: Geneva. http://apps.who.int/iris/bitstream/10665/255016/1/9789241565455-eng.pdf?.... Accessed 25 Apr 2017.
-
- World Health Organization. Guidelines on hepatitis B and C testing. Geneva: 2017. http://apps.who.int/iris/bitstream/10665/254621/1/9789241549981-eng.pdf?.... Accessed 25 Apr 2017.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

