Values, preferences and current hepatitis B and C testing practices in low- and middle-income countries: results of a survey of end users and implementers
- PMID: 29143612
- PMCID: PMC5688454
- DOI: 10.1186/s12879-017-2769-y
Values, preferences and current hepatitis B and C testing practices in low- and middle-income countries: results of a survey of end users and implementers
Abstract
Background: Access to hepatitis B virus (HBV) and hepatitis C virus (HCV) diagnostics remains a key bottleneck in scale-up of access to HBV and HCV treatment, particularly in low- and middle-income countries (LMICs) that lack laboratory resources and skilled personnel. To inform the development of World Health Organization (WHO) testing guidelines on who to test and how to test, we performed a "values and preferences" survey of end users and implementers of hepatitis testing in LMICs on current hepatitis B and C testing practices and acceptability of diagnostic approaches, as well as preferences for the future.
Methods: The survey consisted of a four-part, 28 question online survey tool using SurveyMonkey software. The invitation to participate was sent via email to a network of contacts in hepatitis clinical care, research, advocacy and industry.
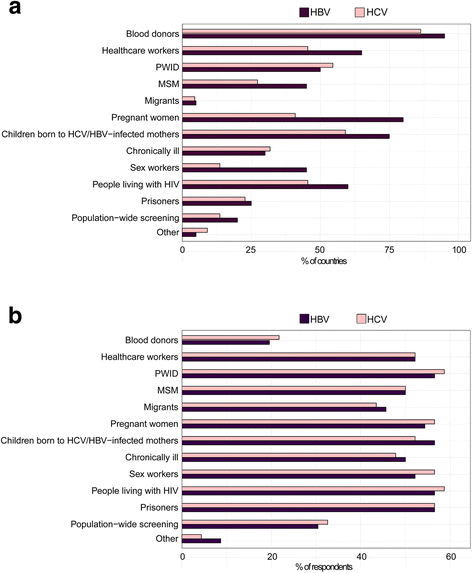
Results: The survey collected responses on current testing practices from 48 respondents in 23 LMICs. Only a small proportion of hepatitis testing is currently funded through government-supported programmes. Most limit their testing programmes to blood donor screening and although testing is recommended in several populations, this is not well implemented. Also, there is still very limited access to virological testing.
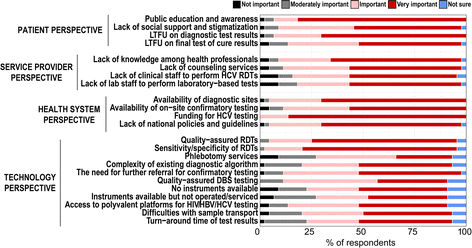
Conclusions: The survey showed that HBV and HCV testing programmes in LMICs are inadequate and/or scarce. Lack of affordable diagnostic tests; lack of funding, public education and awareness; absence of national policies and guidelines; and a dearth of skilled health professionals are the most important barriers to scaling up HBV and HCV diagnosis and treatment.
Conflict of interest statement
Ethics approval and consent to participate
Survey participants were informed that this survey was for research purposes and results will be used to inform the development of WHO guidelines on hepatitis testing. Completion of the emailed survey was taken as de facto consent for participation. The survey was anonymous and did not collect any personal data that could lead to identification of survey participants.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Survey of programmatic experiences and challenges in delivery of hepatitis B and C testing in low- and middle-income countries.BMC Infect Dis. 2017 Nov 1;17(Suppl 1):696. doi: 10.1186/s12879-017-2767-0. BMC Infect Dis. 2017. PMID: 29143609 Free PMC article.
-
Economic evaluation of HCV testing approaches in low and middle income countries.BMC Infect Dis. 2017 Nov 1;17(Suppl 1):697. doi: 10.1186/s12879-017-2779-9. BMC Infect Dis. 2017. PMID: 29143681 Free PMC article. Review.
-
Cost-Effectiveness of Testing and Treatment for Hepatitis B Virus and Hepatitis C Virus Infections: An Analysis by Scenarios, Regions, and Income.Value Health. 2020 Dec;23(12):1552-1560. doi: 10.1016/j.jval.2020.06.015. Epub 2020 Oct 9. Value Health. 2020. PMID: 33248510 Free PMC article.
-
Deceased organ donor screening for HIV, hepatitis B, and hepatitis C viruses: a survey of organ procurement organization practices.Am J Transplant. 2013 Aug;13(8):2186-90. doi: 10.1111/ajt.12260. Epub 2013 May 24. Am J Transplant. 2013. PMID: 23711196
-
Diagnosis of viral hepatitis.Curr Opin HIV AIDS. 2017 May;12(3):302-314. doi: 10.1097/COH.0000000000000370. Curr Opin HIV AIDS. 2017. PMID: 28306597 Free PMC article. Review.
Cited by
-
CRISPR/Cas13a-assisted rapid and portable HBV DNA detection for low-level viremia patients.Emerg Microbes Infect. 2023 Dec;12(1):e2177088. doi: 10.1080/22221751.2023.2177088. Emerg Microbes Infect. 2023. PMID: 36735916 Free PMC article.
-
Hepatitis C Virus Infection in Persons Who Inject Drugs in the Middle East and North Africa: Intervention Strategies.Viruses. 2021 Jul 14;13(7):1363. doi: 10.3390/v13071363. Viruses. 2021. PMID: 34372569 Free PMC article. Review.
-
Retrospective Evaluation of Hepatitis C Awareness in Turkey Through Two Decades.Turk J Gastroenterol. 2021 Jan;32(1):88-96. doi: 10.5152/tjg.2020.19949. Turk J Gastroenterol. 2021. PMID: 33893771 Free PMC article.
-
Diagnostic Performance and Usability of the Genedrive® HCV ID Kit in Two Decentralized Settings in Cameroon and Georgia.Diagnostics (Basel). 2021 Apr 22;11(5):746. doi: 10.3390/diagnostics11050746. Diagnostics (Basel). 2021. PMID: 33921930 Free PMC article.
-
Survey of lived experiences and challenges in hepatitis B management and treatment.BMC Public Health. 2024 Apr 2;24(1):944. doi: 10.1186/s12889-024-18425-w. BMC Public Health. 2024. PMID: 38566070 Free PMC article.
References
-
- World Health Organization. Global Hepatitis Report, 2017. 2017: Geneva. http://apps.who.int/iris/bitstream/10665/255016/1/9789241565455-eng.pdf?...). Accessed 22 June 2017.
-
- Lim SG. HCV management in resource-constrained countries. Hepatol Int. 2017;Feb 21, Epub. doi: 10.1007/s12072-017-9787-0. - PubMed
-
- Zampino R, Sagnelli C, Boemio A, Sagnelli E, Coppola N. Treatment of chronic HBV infection in developing countries. Ann Hepatol. 2016;15:816–23. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

