Diagnostic accuracy of tests to detect hepatitis B surface antigen: a systematic review of the literature and meta-analysis
- PMID: 29143619
- PMCID: PMC5688498
- DOI: 10.1186/s12879-017-2772-3
Diagnostic accuracy of tests to detect hepatitis B surface antigen: a systematic review of the literature and meta-analysis
Abstract
Background: Chronic Hepatitis B Virus (HBV) infection is characterised by the persistence of hepatitis B surface antigen (HBsAg). Expanding HBV diagnosis and treatment programmes into low resource settings will require high quality but inexpensive rapid diagnostic tests (RDTs) in addition to laboratory-based enzyme immunoassays (EIAs) to detect HBsAg. The purpose of this review is to assess the clinical accuracy of available diagnostic tests to detect HBsAg to inform recommendations on testing strategies in 2017 WHO hepatitis testing guidelines.
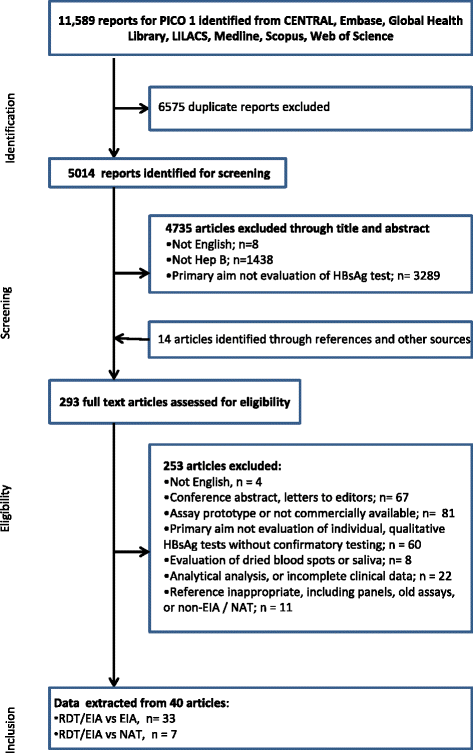
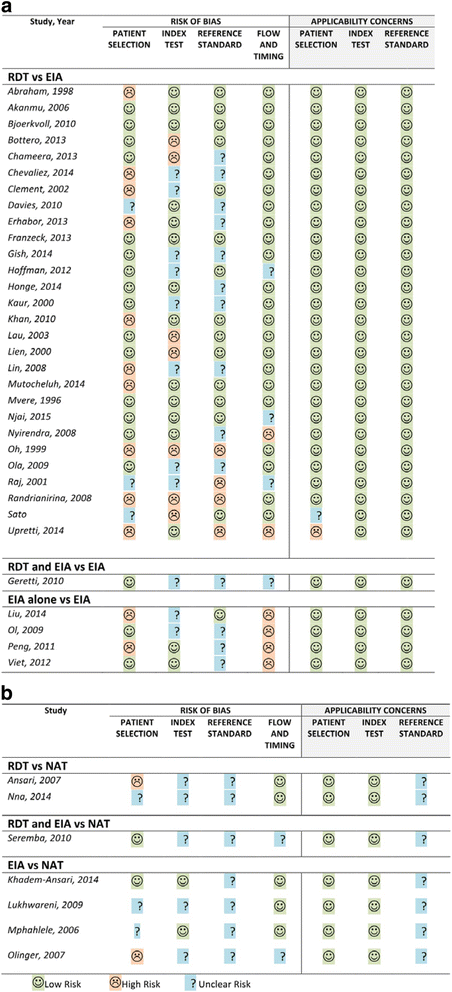
Methods: The systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines using 9 databases. Two reviewers independently extracted data according to a pre-specified plan and evaluated study quality. Meta-analysis was performed. HBsAg diagnostic accuracy of rapid diagnostic tests (RDTs) was compared to enzyme immunoassay (EIA) and nucleic-acid test (NAT) reference standards. Subanalyses were performed to determine accuracy among brands, HIV-status and specimen type.
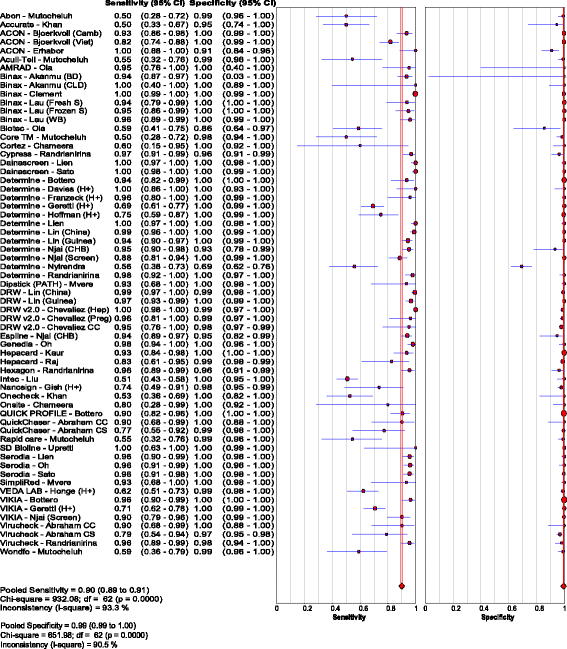
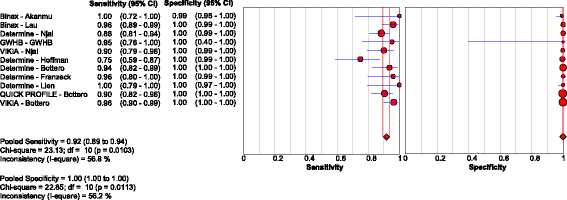
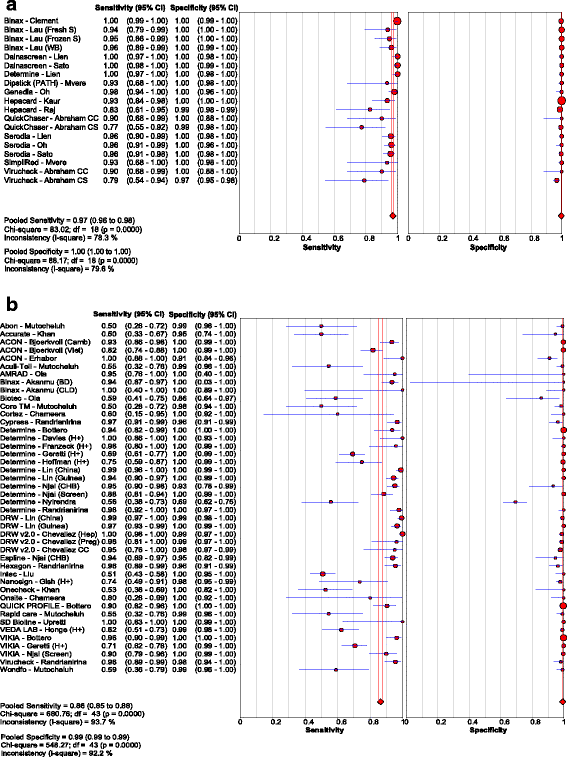
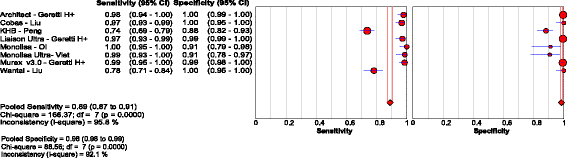
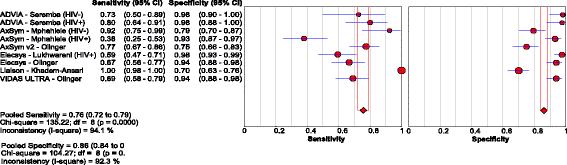
Results: Of the 40 studies that met the inclusion criteria, 33 compared RDTs and/or EIAs against EIAs and 7 against NATs as reference standards. Thirty studies assessed diagnostic accuracy of 33 brands of RDTs in 23,716 individuals from 23 countries using EIA as the reference standard. The pooled sensitivity and specificity were 90.0% (95% CI: 89.1, 90.8) and 99.5% (95% CI: 99.4, 99.5) respectively, but accuracy varied widely among brands. Accuracy did not differ significantly whether serum, plasma, venous or capillary whole blood was used. Pooled sensitivity of RDTs in 5 studies of HIV-positive persons was lower at 72.3% (95% CI: 67.9, 76.4) compared to that in HIV-negative persons, but specificity remained high. Five studies evaluated 8 EIAs against a chemiluminescence immunoassay reference standard with a pooled sensitivity and specificity of 88.9% (95% CI: 87.0, 90.6) and 98.4% (95% CI: 97.8, 98.8), respectively. Accuracy of both RDTs and EIAs using a NAT reference were generally lower, especially amongst HIV-positive cohorts.
Conclusions: HBsAg RDTs have good sensitivity and excellent specificity compared to laboratory immunoassays as a reference standard. Sensitivity of HBsAg RDTs may be lower in HIV infected individuals.
Keywords: CMIA; Diagnostic accuracy; Diagnostic tests; Enzyme immunoassays; Hepatitis B virus; MEIA; Rapid diagnostic tests.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
-
- World Health Organization. Global hepatitis report 2017. 2017: Geneva. http://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/
-
- Naghavi M, Wang H, Lozano R, Davis A, Liang X, Zhou M, et al. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2015;385(9963):117–171. doi: 10.1016/S0140-6736(14)61682-2. - DOI - PMC - PubMed
-
- World Health Organization. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. 2015: Geneva. http://www.who.int/hiv/pub/hepatitis/hepatitis-b-guidelines/en/ - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

