Trends in hepatitis B virus testing practices and management in HIV clinics across sub-Saharan Africa
- PMID: 29143625
- PMCID: PMC5688463
- DOI: 10.1186/s12879-017-2768-z
Trends in hepatitis B virus testing practices and management in HIV clinics across sub-Saharan Africa
Abstract
Background: Approximately 8% of HIV-infected individuals are co-infected with hepatitis B virus (HBV) in sub-Saharan Africa (SSA). Knowledge of HBV status is important to guide optimal selection of antiretroviral therapy (ART) and monitor/prevent liver-related complications. We describe changes in testing practices and management of HBV infection over a 3-year period in HIV clinics across SSA.
Methods: A medical chart review was conducted in large urban HIV treatment centers in Côte d'Ivoire (3 sites), Benin, Burkina Faso, Cameroon, Kenya, Senegal, South Africa, Togo, Uganda and Zambia (1 site each). Of the patients who started ART between 2010 and 2012, 100 per year were randomly selected from each clinic. Demographic, clinical and laboratory information as well as individual treatment histories were collected using a standardized questionnaire. We examined changes over time in the proportion of patients screened for HBV infection (HBV surface antigen [HBsAg]-positivity), identified predictors of HBV testing using logistic regression, and assessed the proportion of patients initiating a tenofovir (TDF)-containing ART regimen.
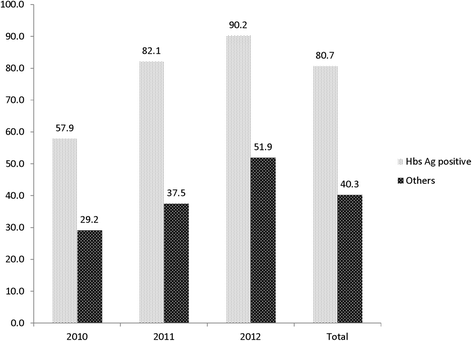
Results: Overall, 3579 charts of patients initiating ART (64.4% female, median age 37 years) were reviewed in 12 clinics. The proportion of patients screened for HBsAg increased from 17.8% in 2010 to 24.4% in 2012 overall, and ranged from 0.7% in Kenya to 96% in South Africa. In multivariable analyses, age and region were associated with HBsAg screening. Among 759 individuals tested, 88 (11.6%; 95% confidence interval [CI] 9.4-14.1) were HBV-infected, of whom 71 (80.7%) received a TDF-containing ART regimen. HBsAg-positive individuals were twice as likely to receive a TDF-containing first-line ART regimen compared to HBsAg-negative patients (80.7% vs. 40.3%, p < 0.001). The proportion of patients on TDF-containing ART increased from 57.9% in 2010 to 90.2% in 2012 in HIV/HBV-co-infected patients (Chi-2 test for trend: p = 0.01). Only 114 (5.0%) patients were screened for anti-HCV antibodies and one of them (0.9%, 95% CI 0.02-4.79) had a confirmed HCV infection.
Conclusions: The systematic screening for HBV infection in HIV-positive patients before ART initiation was limited in most African countries and its uptake varied widely across clinics. Overall, the prescription of TDF increased over time, with 90% of HIV/HBV-coinfected patients receiving this drug in 2012.
Conflict of interest statement
Ethics approval and consent to participate
Ethical permission for participation in IeDEAwas given by the national ethics committee of each participating country and all patients were informed and had to give their written consent before being included.
Specific ethics approval for this study was sought from the following institutions:
“Comité d’Ethique pour la Recherche en Santé au Bénin (CNERB)” in Benin, “Comité d’Ethique pour la Recherche en Santé au Burkina Faso” (CERS_BF) in Burkina-Faso, “Comité National d’Ethique et la Recherche en Santé” (CNER_CI) in Côte d’Ivoire, the “Comité de Bioéthique pour la Recherche en Santé (CBRS)” in Togo, “Comité National d’Ethique pour la Recherche en Santé au Senegal” (CNERS) Senegal, « Health Research Ethics Committee » of Stellenbosch University, South Africa.
Consent for publication
Not applicable.
Competing interests
The Authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Stabinski L, O'Connor S, Barnhart M, Kahn RJ, Hamm TE. Prevalence of HIV and hepatitis B virus co-infection in sub-Saharan Africa and the potential impact and program feasibility of hepatitis B surface antigen screening in resource-limited settings. J Acquir Immune Defic Syndr. 2015;68(Suppl 3):S274–S285. doi: 10.1097/QAI.0000000000000496. - DOI - PMC - PubMed
-
- Wandeler G, Gsponer T, Bihl F, Bernasconi E, Cavassini M, Kovari H, Schmid P, Battegay M, Calmy A, Egger M, et al. Hepatitis B virus infection is associated with impaired immunological recovery during antiretroviral therapy in the Swiss HIV cohort study. J Infect Dis. 2013;208(9):1454–1458. doi: 10.1093/infdis/jit351. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

