Primary Cilium-Dependent Signaling Mechanisms
- PMID: 29143784
- PMCID: PMC5713242
- DOI: 10.3390/ijms18112272
Primary Cilium-Dependent Signaling Mechanisms
Abstract
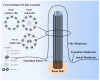
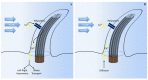
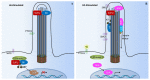
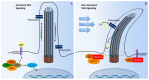
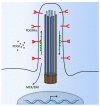
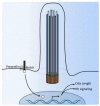
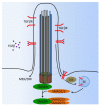
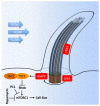
Primary cilia are hair-like organelles and play crucial roles in vertebrate development, organogenesis, health, and many genetic disorders. A primary cilium is a mechano-sensory organelle that responds to mechanical stimuli in the micro-environment. A cilium is also a chemosensor that senses chemical signals surrounding a cell. The overall function of a cilium is therefore to act as a communication hub to transfer extracellular signals into intracellular responses. Although intracellular calcium has been one of the most studied signaling messengers that transmit extracellular signals into the cells, calcium signaling by various ion channels remains a topic of interest in the field. This may be due to a broad spectrum of cilia functions that are dependent on or independent of utilizing calcium as a second messenger. We therefore revisit and discuss the calcium-dependent and calcium-independent ciliary signaling pathways of Hedgehog, Wnt, PDGFR, Notch, TGF-β, mTOR, OFD1 autophagy, and other GPCR-associated signaling. All of these signaling pathways play crucial roles in various cellular processes, such as in organ and embryonic development, cardiac functioning, planar cell polarity, transactivation, differentiation, the cell cycle, apoptosis, tissue homeostasis, and the immune response.
Keywords: calcium; cilioplasm; cytoplasm; sensory function; signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

