Next-generation pacemakers: from small devices to biological pacemakers
- PMID: 29143810
- PMCID: PMC6261336
- DOI: 10.1038/nrcardio.2017.165
Next-generation pacemakers: from small devices to biological pacemakers
Abstract
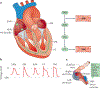
Electrogenesis in the heart begins in the sinoatrial node and proceeds down the conduction system to originate the heartbeat. Conduction system disorders lead to slow heart rates that are insufficient to support the circulation, necessitating implantation of electronic pacemakers. The typical electronic pacemaker consists of a subcutaneous generator and battery module attached to one or more endocardial leads. New leadless pacemakers can be implanted directly into the right ventricular apex, providing single-chamber pacing without a subcutaneous generator. Modern pacemakers are generally reliable, and their programmability provides options for different pacing modes tailored to specific clinical needs. Advances in device technology will probably include alternative energy sources and dual-chamber leadless pacing in the not-too-distant future. Although effective, current electronic devices have limitations related to lead or generator malfunction, lack of autonomic responsiveness, undesirable interactions with strong magnetic fields, and device-related infections. Biological pacemakers, generated by somatic gene transfer, cell fusion, or cell transplantation, provide an alternative to electronic devices. Somatic reprogramming strategies, which involve transfer of genes encoding transcription factors to transform working myocardium into a surrogate sinoatrial node, are furthest along in the translational pipeline. Even as electronic pacemakers become smaller and less invasive, biological pacemakers might expand the therapeutic armamentarium for conduction system disorders.
Conflict of interest statement
Competing interests statement
The authors declare no competing interests.
Figures
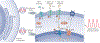
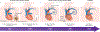
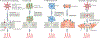
References
-
- Miranda JO, Ramalho C, Henriques-Coelho T & Areias JC Fetal programming as a predictor of adult health or disease: the need to reevaluate fetal heart function. Heart Fail. Rev. 22, 861–877 (2017). - PubMed
-
- Marban E Cardiac channelopathies. Nature 415, 213–218 (2002). - PubMed
-
- Bers DM Cardiac excitation-contraction coupling. Nature 415, 198–205 (2002). - PubMed
-
- Crick SJ et al. Innervation of the human cardiac conduction system. A quantitative immunohistochemical and histochemical study. Circulation 89, 1697–1708 (1994). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

