Effect of reagents used during detection and quantification of Ascaris suum in environmental samples on egg viability
- PMID: 29144297
- PMCID: PMC7797636
- DOI: 10.2166/wst.2017.324
Effect of reagents used during detection and quantification of Ascaris suum in environmental samples on egg viability
Abstract
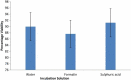
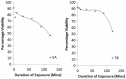
Soil-transmitted helminths (STHs) are a major health concern globally. Infection is mostly through contact with contaminated water, food or soil. Therefore to break the cycle of viable transmission STH eggs must be quantitatively detected in the environment. The effect of different reagents on the viability of Ascaris suum eggs during laboratory detection and quantification was assessed and different incubation solutions compared. Sulphuric acid gave a slightly higher recovery percentage of viable eggs (91.2%) than distilled water (90.0%) and 0.5% formalin (87.6%), although the difference was not statistically significant (p > 0.05). Acetoacetic acid, ethyl acetate, ammonium bicarbonate, zinc sulphate, magnesium sulphate and Tween 80, are reagents widely used in test protocols for the detection and quantification of STH eggs. Eggs were exposed to these reagents for different time durations. Acetoacetic acid resulted in the highest loss of viability (3.4 ± 0.7% viable), while magnesium sulphate resulted in the least effect (88.5 ± 1.2% viable). In conclusion the use of the selected reagents in the detection of these eggs was found to affect the viability of exposed eggs, especially during prolonged exposures. Therefore we recommended that eggs be exposed for ≤5 minutes, to reduce the risk of viability loss.
Figures




References
-
- Amahmid O. & Bouhoum K.. 2005. Assessment of the health hazards associated with wastewater reuse: transmission of geohelminthic infections (Marrakech, Morocco). International Journal of Environmental Health Research 15 (2), 127–133. - PubMed
-
- Angelakis A. N. & Bontoux L.. 2001. Wastewater reclamation and reuse in Eureau countries. Water Policy 3 (1), 47–59.
-
- Ayres R. M. & Mara D. D.. 1996. Analysis of Wastewater for Use in Agriculture: A Laboratory Manual of Parasitological and Bacteriological Techniques. World Health Organization , Geneva, Switzerland.
-
- Bornay-Llinares F. J., Navarro-i-Martínez L., García-Orenes F., Araez H., Pérez-Murcia M. D. & Moral R.. 2006. Detection of intestinal parasites in pigslurry: a preliminary study from five farms in Spain. Livestock Science 102 (3), 237–242.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

