Treatment Approaches to Moderate to Severe Psoriasis
- PMID: 29144382
- PMCID: PMC5713395
- DOI: 10.3390/ijms18112427
Treatment Approaches to Moderate to Severe Psoriasis
Abstract
Psoriasis is a common disease, which has a considerable impact on patients and the health care system. Treatment approaches to the disease may be various because some issues are not definitely addressed. Moreover, the therapeutic paradigms are continuously changing because of the recent approval of new treatments for psoriasis such as interleukin (IL)-17 inhibitors and apremilast. In this review, the factors influencing psoriasis severity, the indications for systemic treatments, the overall parameters to be considered in the treatment choice, life style interventions, and the recommendations for the use, screening, and monitoring of systemic therapies available including acitretin, cyclosporine, methotrexate, apremilast, adalimumab, etanercept, infliximab, secukinumab, ixekizumab, and ustekinumab are discussed. Finally, treatment approaches in special patient populations including children, the elderly, pregnant women, patients with a history of neoplasm, and candidates for surgical procedures are reported.
Keywords: biologics; psoriasis; therapy.
Conflict of interest statement
Paolo Gisondi served as consultant and/or as speaker for AbbVie, Abiogen, Celgene, Eli-Lilly, Janssen, Leo Pharma, MSD, Novartis, Pfizer, Pierre Fabre, Sanofi. Micol Del Giglio declares no conflict of interest. Giampiero Girolomoni served as consultant and/or as speaker for AbbVie, Abiogen, Allmirall, Amgen, Bayer, Biogen, Boehringer Ingelheim, Celgene, Eli-Lilly, Galderma, Hospira, Janssen, Leo Pharma, Merck, MSD, Mundipharma, Novartis, Otsuka, Pfizer, Pierre Fabre, Regeneron, Sandoz, Sanofi, Sun Pharma.
Figures
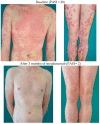
References
-
- Robinson A., Kardos M., Kimball A.B. Physician Global Assessment (PGA) and Psoriasis Area and Severity Index (PASI): Why do both? A systematic analysis of randomized controlled trials of biologic agents for moderate to severe plaque psoriasis. J. Am. Acad. Dermatol. 2012;66:369–375. doi: 10.1016/j.jaad.2011.01.022. - DOI - PubMed
-
- Nast A., Gisondi P., Ormerod A.D., Saiag P., Smith C., Spuls P.I., Arenberger P., Bachelez H., Barker J., Dauden E., et al. European S3-Guidelines on the systemic treatment of psoriasis vulgaris—Update 2015--Short version—EDF in cooperation with EADV and IPC. J. Eur. Acad. Dermatol. Venereol. 2015;29:2277–2294. doi: 10.1111/jdv.13354. - DOI - PubMed
-
- Mrowietz U., Kragballe K., Reich K., Spuls P., Griffiths C.E., Nast A., Franke J., Antoniou C., Arenberger P., Balieva F., et al. Definition of treatment goals for moderate to severe psoriasis: A European consensus. Arch. Dermatol. Res. 2011;303:1–10. doi: 10.1007/s00403-010-1080-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

