Synthesis, Characterization and Biological Activities of Biopolymeric Schiff Bases Prepared with Chitosan and Salicylaldehydes and Their Pd(II) and Pt(II) Complexes
- PMID: 29144424
- PMCID: PMC6150178
- DOI: 10.3390/molecules22111987
Synthesis, Characterization and Biological Activities of Biopolymeric Schiff Bases Prepared with Chitosan and Salicylaldehydes and Their Pd(II) and Pt(II) Complexes
Abstract
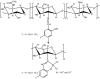
In an attempt to enhance chitosan biological activities, biopolymeric Schiff bases of chitosan and different salicylaldehydes and their palladium(II) and platinum(II) complexes were synthesized and tested. The chemical structures of these derivatives were characterized using ¹H-NMR, FTIR spectroscopy and XPRD. Thermal analysis was done through TGA/DTG-DTA. Electronic absorption spectra and surface morphologies were analyzed by SEM-EDAX. Chitosan and its derivatives were evaluated for their in vitro antimicrobial activity against two common bacterial and fungal plant pathogens Pseudomonas syringae pv. tomato and Fusarium graminearum, respectively, and for their antitumor activity against a human breast cancer cell line (MCF-7). It was found that, compared to the nonmodified chitosan, chitosan modified with Schiff bases and their complexes was highly toxic against the MCF-7 cell line and had antibacterial effects against P. syringea. However, the modified chitosan derivatives had less pronounced antifungal effects against F. graminearum compared to the nonmodified chitosan, suggesting different modes of action.
Keywords: Schiff bases; antimicrobial; antitumor; chitosan; complexes.
Conflict of interest statement
The authors declare no conflicts of interests.
Figures




Similar articles
-
New series of metal complexes by amphiphilic biopolymeric Schiff bases from modified chitosans: Preparation, characterization and effect of molecular weight on its biological applications.Int J Biol Macromol. 2020 Feb 15;145:417-428. doi: 10.1016/j.ijbiomac.2019.12.153. Epub 2019 Dec 20. Int J Biol Macromol. 2020. PMID: 31870879
-
Characterization, solubility and biological activity of amphihilic biopolymeric Schiff bases synthesized using chitosans.Carbohydr Polym. 2019 Sep 15;220:1-11. doi: 10.1016/j.carbpol.2019.05.037. Epub 2019 May 11. Carbohydr Polym. 2019. PMID: 31196526
-
Synthesis, characterization and biological activity of Cu(II), Ni(II) and Zn(II) complexes of biopolymeric Schiff bases of salicylaldehydes and chitosan.Int J Biol Macromol. 2017 Feb;95:168-176. doi: 10.1016/j.ijbiomac.2016.10.109. Epub 2016 Nov 13. Int J Biol Macromol. 2017. PMID: 27851928
-
Schiff Bases and their Metal Complexes as Potential Anticancer Candidates: A Review of Recent Works.Anticancer Agents Med Chem. 2019;19(15):1786-1795. doi: 10.2174/1871520619666190227171716. Anticancer Agents Med Chem. 2019. PMID: 30827264 Review.
-
Chitosan-Based Schiff Bases (CSBs) and Their Metal Complexes: Promising Antimicrobial Agents.Molecules. 2025 Jan 7;30(2):207. doi: 10.3390/molecules30020207. Molecules. 2025. PMID: 39860077 Free PMC article. Review.
Cited by
-
Magnetically recoverable Fe3O4@chitosan@Ni2B: a bio-based catalyst for one-pot green and efficient synthesis of tetrahydrobenzo[b]pyrans.Nanoscale Adv. 2025 May 9;7(12):3701-3721. doi: 10.1039/d4na01020e. eCollection 2025 Jun 10. Nanoscale Adv. 2025. PMID: 40352460 Free PMC article.
-
Schiff Bases: A Captivating Scaffold with Potential Anticonvulsant Activity.Mini Rev Med Chem. 2024;24(18):1632-1650. doi: 10.2174/0113895575302197240408121537. Mini Rev Med Chem. 2024. PMID: 38629363 Review.
-
Cytotoxicity Induced by Newly Synthesized Palladium (II) Complexes Lead to the Death of MCF-7 and MDA-MB-435 Cancer Cell Lines.Adv Pharm Bull. 2023 Jan;13(1):160-169. doi: 10.34172/apb.2023.017. Epub 2021 Oct 10. Adv Pharm Bull. 2023. PMID: 36721806 Free PMC article.
-
Tuning Antioxidant Function through Dynamic Design of Chitosan-Based Hydrogels.Gels. 2024 Oct 13;10(10):655. doi: 10.3390/gels10100655. Gels. 2024. PMID: 39451308 Free PMC article.
-
Advances in Coordination Chemistry of Schiff Base Complexes: A Journey from Nanoarchitectonic Design to Biomedical Applications.Top Curr Chem (Cham). 2025 Feb 3;383(1):8. doi: 10.1007/s41061-025-00489-w. Top Curr Chem (Cham). 2025. PMID: 39900838 Review.
References
-
- Rinaudo M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006;31:603–632. doi: 10.1016/j.progpolymsci.2006.06.001. - DOI
-
- Xia W., Liu P., Zhang J., Chen J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocoll. 2011;25:170–179. doi: 10.1016/j.foodhyd.2010.03.003. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

