Progress in Rubella and Congenital Rubella Syndrome Control and Elimination - Worldwide, 2000-2016
- PMID: 29145358
- PMCID: PMC5726242
- DOI: 10.15585/mmwr.mm6645a4
Progress in Rubella and Congenital Rubella Syndrome Control and Elimination - Worldwide, 2000-2016
Abstract
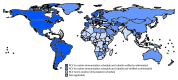
Although rubella virus infection usually causes a mild fever and rash illness in children and adults, infection during pregnancy, especially during the first trimester, can result in miscarriage, fetal death, stillbirth, or infants with a constellation of congenital malformations known as congenital rubella syndrome (CRS) (1). Rubella is a leading vaccine-preventable cause of birth defects. Preventing these adverse pregnancy outcomes is the focus of rubella vaccination programs. In 2011, the World Health Organization (WHO) updated guidance on the preferred strategy for introduction of rubella-containing vaccine (RCV) into national immunization schedules and recommended an initial vaccination campaign, usually targeting children aged 9 months-14 years (1). The Global Vaccine Action Plan 2011-2020 (GVAP), endorsed by the World Health Assembly in 2012, includes goals to eliminate rubella in at least five of the six WHO regions by 2020 (2). This report updates a previous report (3) and summarizes global progress toward rubella and CRS control and elimination from 2000 to 2016. As of December 2016, 152 (78%) of 194 countries had introduced RCV into the national immunization schedule, representing an increase of 53 countries since 2000, including 20 countries that introduced RCV after 2012.
Conflict of interest statement
Figures
References
-
- World Health Organization. Rubella vaccines: WHO position paper. Wkly Epidemiol Rec 2011;86:301–16. - PubMed
-
- World Health Organization. Global vaccine action plan. Geneva, Switzerland: World Health Organization; 2012. http://apps.who.int/iris/bitstream/10665/78141/1/9789241504980_eng.pdf?ua=1
-
- World Health Organization. Immunization surveillance, assessment, and monitoring. Data statistics and graphics by subject. Geneva, Switzerland: World Health Organization; 2017. http://www.who.int/immunization/monitoring_surveillance/data/en/
-
- World Health Organization. WHO-recommended standards for surveillance of selected vaccine-preventable diseases. Geneva, Switzerland: World Health Organization ; 2003. http://apps.who.int/iris/bitstream/10665/68334/1/WHO_V-B_03.01_eng.pdf?ua=1
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials