A new approach by optical coherence tomography for elucidating biofilm formation by emergent Candida species
- PMID: 29145445
- PMCID: PMC5690619
- DOI: 10.1371/journal.pone.0188020
A new approach by optical coherence tomography for elucidating biofilm formation by emergent Candida species
Abstract
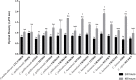
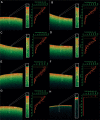
The majority of microorganisms present a community lifestyle, establishing biofilm ecosystems. However, little is known about its formation in emergent Candida species involved in catheter-related infections. Thus, various techniques may be used in the biofilm detection to elucidate structure and clinical impact. In this context, we report the ability of emergent Candida species (Candida haemulonii, C. lusitaniae, C. pelliculosa, C.guilliermondii, C. famata and C. ciferrii) on developing well structured biofilms with cell viability and architecture, using optical coherence tomography (OCT). This new approach was compared with XTT analyses and Scanning Electron Microscopy (SEM). A positive correlation between oxidative activity (XTT) and OCT results (r = 0.8752, p < 0.0001) was observed. SEM images demonstrated cells attachment, multilayer and morphologic characteristics of the biofilm structure. C. lusitaniae was the emergent species which revealed the highest scattering extension length and oxidative metabolism when evaluated by OCT and XTT methods, respectively. Herein, information on C. ciferri biofilm structure were presented for the first time. The OCT results are independently among Candida strains and no species-specific pattern was observed. Our findings strongly contribute for clinical management based on the knowledge of pathogenicity mechanisms involving emergent yeasts.
Conflict of interest statement
Figures


Similar articles
-
Biofilm production and evaluation of antifungal susceptibility amongst clinical Candida spp. isolates, including strains of the Candida parapsilosis complex.Med Mycol. 2011 Apr;49(3):253-62. doi: 10.3109/13693786.2010.530032. Epub 2010 Nov 2. Med Mycol. 2011. PMID: 21039308
-
Characterization of biofilms formed by Candida parapsilosis, C. metapsilosis, and C. orthopsilosis.Int J Med Microbiol. 2010 Apr;300(4):265-70. doi: 10.1016/j.ijmm.2009.09.001. Epub 2009 Nov 22. Int J Med Microbiol. 2010. PMID: 19932053
-
Assessment of in vitro biofilm formation by Candida species isolates from vulvovaginal candidiasis and ultrastructural characteristics.Micron. 2012 Feb;43(2-3):497-502. doi: 10.1016/j.micron.2011.09.013. Epub 2011 Sep 29. Micron. 2012. PMID: 22001373
-
Optical coherence tomography in biofilm research: A comprehensive review.Biotechnol Bioeng. 2017 Jul;114(7):1386-1402. doi: 10.1002/bit.26283. Epub 2017 Mar 23. Biotechnol Bioeng. 2017. PMID: 28266013 Review.
-
Methods for obtaining reliable and reproducible results in studies of Candida biofilms formed in vitro.Mycoses. 2013 Nov;56(6):614-22. doi: 10.1111/myc.12092. Epub 2013 May 27. Mycoses. 2013. PMID: 23710618 Review.
Cited by
-
Testing Anti-Biofilm Polymeric Surfaces: Where to Start?Int J Mol Sci. 2019 Aug 3;20(15):3794. doi: 10.3390/ijms20153794. Int J Mol Sci. 2019. PMID: 31382580 Free PMC article. Review.
-
Intra-colony channels in E. coli function as a nutrient uptake system.ISME J. 2020 Oct;14(10):2461-2473. doi: 10.1038/s41396-020-0700-9. Epub 2020 Jun 17. ISME J. 2020. PMID: 32555430 Free PMC article.
-
Candida lusitaniae: Biology, Pathogenicity, Virulence Factors, Diagnosis, and Treatment.Infect Drug Resist. 2022 Aug 31;15:5121-5135. doi: 10.2147/IDR.S383785. eCollection 2022. Infect Drug Resist. 2022. PMID: 36068831 Free PMC article. Review.
-
Antifungal Effect and Inhibition of the Virulence Mechanism of D-Limonene against Candida parapsilosis.Molecules. 2022 Dec 14;27(24):8884. doi: 10.3390/molecules27248884. Molecules. 2022. PMID: 36558017 Free PMC article.
-
WMR Peptide as Antifungal and Antibiofilm against Albicans and Non-Albicans Candida Species: Shreds of Evidence on the Mechanism of Action.Int J Mol Sci. 2022 Feb 15;23(4):2151. doi: 10.3390/ijms23042151. Int J Mol Sci. 2022. PMID: 35216270 Free PMC article.
References
-
- Kullberg BJ, and Arendrup MC. Invasive Candidiasis. N. Engl. J. Med. 2015; 15: 373. - PubMed
-
- Silva FC, Viana VO, Araújo BP, Campos LANL, and Rosa LP. Prevalence of Candida yeasts in oral samples from children with AIDS and children exposed and not exposed to HIV served by SUS in the state of Bahia, Brazil. Rev. Gaúch. Odontol. 2015; 63: 7–12.
-
- Li Y, Xu W, Jiang Z, Gao Y, Pang Y, Li L, et al. Neutropenia and invasive fungal infection in patients with hematological malignancies treated with chemotherapy: a multicenter, prospective, non-interventional study in China. Tumour Biol. 2014; 35: 5869–5876. doi: 10.1007/s13277-014-1777-4 - DOI - PubMed
-
- Donlan RM. Biofilms: microbial life on surfaces. Emerg. Infect. Dis. 2002; 8: 881–890. doi: 10.3201/eid0809.020063 - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

