TRAIL attenuates RANKL-mediated osteoblastic signalling in vascular cell mono-culture and co-culture models
- PMID: 29145460
- PMCID: PMC5690591
- DOI: 10.1371/journal.pone.0188192
TRAIL attenuates RANKL-mediated osteoblastic signalling in vascular cell mono-culture and co-culture models
Abstract
Background and objectives: Vascular calcification (VC) is a major risk factor for elevated cardiovascular morbidity/mortality. Underlying this process is osteoblastic signalling within the vessel wall involving complex and interlinked roles for receptor-activator of nuclear factor-κB ligand (RANKL), osteoprotegerin (OPG), and tumour necrosis factor-related apoptosis-inducing ligand (TRAIL). RANKL promotes vascular cell osteoblastic differentiation, whilst OPG acts as a neutralizing decoy receptor for RANKL (and TRAIL). With respect to TRAIL, much recent evidence points to a vasoprotective role for this ligand, albeit via unknown mechanisms. In order to shed more light on TRAILs vasoprotective role therefore, we employed in vitro cell models to test the hypothesis that TRAIL can counteract the RANKL-mediated signalling that occurs between the vascular cells that comprise the vessel wall.
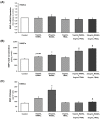
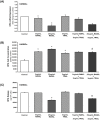
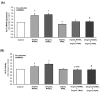
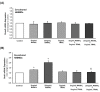
Methods and results: Human aortic endothelial and smooth muscle cell mono-cultures (HAECs, HASMCs) were treated with RANKL (0-25 ng/mL ± 5 ng/mL TRAIL) for 72 hr. Furthermore, to better recapitulate the paracrine signalling that exists between endothelial and smooth muscle cells within the vessel wall, non-contact transwell HAEC:HASMC co-cultures were also employed and involved RANKL treatment of HAECs (±TRAIL), subsequently followed by analysis of pro-calcific markers in the underlying subluminal HASMCs. RANKL elicited robust osteoblastic signalling across both mono- and co-culture models (e.g. increased BMP-2, alkaline phosphatase/ALP, Runx2, and Sox9, in conjunction with decreased OPG). Importantly, several RANKL actions (e.g. increased BMP-2 release from mono-cultured HAECs or increased ALP/Sox9 levels in co-cultured HASMCs) could be strongly blocked by co-incubation with TRAIL. In summary, this paper clearly demonstrates that RANKL can elicit pro-osteoblastic signalling in HAECs and HASMCs both directly and across paracrine signalling axes. Moreover, within these contexts we present clear evidence that TRAIL can block several key signalling actions of RANKL in vascular cells, providing further evidence of its vasoprotective potential.
Conflict of interest statement
Figures


Similar articles
-
Activation of the non-canonical NF-κB/p52 pathway in vascular endothelial cells by RANKL elicits pro-calcific signalling in co-cultured smooth muscle cells.Cell Signal. 2018 Jul;47:142-150. doi: 10.1016/j.cellsig.2018.04.004. Epub 2018 Apr 17. Cell Signal. 2018. PMID: 29678621 Free PMC article.
-
RANKL treatment of vascular endothelial cells leading to paracrine pro-calcific signaling involves ROS production.Mol Cell Biochem. 2020 Jan;464(1-2):111-117. doi: 10.1007/s11010-019-03653-1. Epub 2019 Nov 14. Mol Cell Biochem. 2020. PMID: 31724123
-
RANKL promotes osteoblastic activity in vascular smooth muscle cells by upregulating endothelial BMP-2 release.Int J Biochem Cell Biol. 2016 Aug;77(Pt A):171-180. doi: 10.1016/j.biocel.2016.06.009. Epub 2016 Jun 23. Int J Biochem Cell Biol. 2016. PMID: 27339040
-
Vascular calcification in type-2 diabetes and cardiovascular disease: Integrative roles for OPG, RANKL and TRAIL.Vascul Pharmacol. 2016 Jul;82:30-40. doi: 10.1016/j.vph.2016.02.003. Epub 2016 Feb 24. Vascul Pharmacol. 2016. PMID: 26924459 Review.
-
Role of osteoprotegerin and its ligands and competing receptors in atherosclerotic calcification.J Investig Med. 2006 Nov;54(7):395-401. doi: 10.2310/6650.2006.06019. J Investig Med. 2006. PMID: 17169261 Review.
Cited by
-
Endothelial TIE2 Mutation Induced Contraction Deficiency of Vascular Smooth Muscle Cells via Phenotypic Transition Regulation in Venous Malformations.Int J Med Sci. 2025 May 7;22(10):2518-2532. doi: 10.7150/ijms.102700. eCollection 2025. Int J Med Sci. 2025. PMID: 40386049 Free PMC article.
-
Activation of the non-canonical NF-κB/p52 pathway in vascular endothelial cells by RANKL elicits pro-calcific signalling in co-cultured smooth muscle cells.Cell Signal. 2018 Jul;47:142-150. doi: 10.1016/j.cellsig.2018.04.004. Epub 2018 Apr 17. Cell Signal. 2018. PMID: 29678621 Free PMC article.
-
Metabolic and densitometric correlation between atherosclerotic plaque and trabecular bone: an 18F-Natrium-Fluoride PET/CT study.Am J Nucl Med Mol Imaging. 2018 Dec 20;8(6):387-396. eCollection 2018. Am J Nucl Med Mol Imaging. 2018. PMID: 30697458 Free PMC article.
-
Hyperuricemia remodels the serum proteome toward a higher inflammatory state.iScience. 2023 Sep 14;26(10):107909. doi: 10.1016/j.isci.2023.107909. eCollection 2023 Oct 20. iScience. 2023. PMID: 37810213 Free PMC article.
-
RANKL treatment of vascular endothelial cells leading to paracrine pro-calcific signaling involves ROS production.Mol Cell Biochem. 2020 Jan;464(1-2):111-117. doi: 10.1007/s11010-019-03653-1. Epub 2019 Nov 14. Mol Cell Biochem. 2020. PMID: 31724123
References
-
- Abedin M, Tintut Y, Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol. 2004; 24: 1161–1170. doi: 10.1161/01.ATV.0000133194.94939.42 - DOI - PubMed
-
- Wexler L, Brundage B, Crouse J, Detrano R, Fuster V, Maddahi J, et al. Coronary artery calcification: pathophysiology, epidemiology, imaging methods, and clinical implications. A statement for health professionals from the American Heart Association. Writing Group. Circulation. 1996; 94: 1175–1192. - PubMed
-
- Demer LL, Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation 2008; 117: 2938–2948. doi: 10.1161/CIRCULATIONAHA.107.743161 - DOI - PMC - PubMed
-
- Qunibi WY, Abouzahr F, Mizani MR, Nolan CR, Arya R, Hunt KJ. Cardiovascular calcification in Hispanic Americans (HA) with chronic kidney disease (CKD) due to type 2 diabetes. Kidney Int. 2005: 68; 271–277. doi: 10.1111/j.1523-1755.2005.00402.x - DOI - PubMed
-
- Sakaguchi M, Hasegawa T, Ehara S, Matsumoto K, Mizutani K, Iguchi T, et al. New insights into spotty calcification and plaque rupture in acute coronary syndrome: an optical coherence tomography study. Heart Vessels. 2016; 31: 1915–1922. doi: 10.1007/s00380-016-0820-3 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

