Adenosine, lidocaine and Mg2+ (ALM) fluid therapy attenuates systemic inflammation, platelet dysfunction and coagulopathy after non-compressible truncal hemorrhage
- PMID: 29145467
- PMCID: PMC5690633
- DOI: 10.1371/journal.pone.0188144
Adenosine, lidocaine and Mg2+ (ALM) fluid therapy attenuates systemic inflammation, platelet dysfunction and coagulopathy after non-compressible truncal hemorrhage
Abstract
Background: Systemic inflammation and coagulopathy are major drivers of injury progression following hemorrhagic trauma. Our aim was to examine the effect of small-volume 3% NaCl adenosine, lidocaine and Mg2+ (ALM) bolus and 0.9% NaCl/ALM 'drip' on inflammation and coagulation in a rat model of hemorrhagic shock.
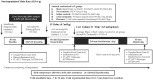
Methods: Sprague-Dawley rats (429±4 g) were randomly assigned to: 1) shams, 2) no-treatment, 3) saline-controls, 4) ALM-therapy, and 5) Hextend®. Hemorrhage was induced in anesthetized-ventilated animals by liver resection (60% left lateral lobe and 50% medial lobe). After 15 min, a bolus of 3% NaCl ± ALM (0.7 ml/kg) was administered intravenously (Phase 1) followed 60 min later by 4 hour infusion of 0.9% NaCl ± ALM (0.5 ml/kg/hour) with 1-hour monitoring (Phase 2). Plasma cytokines were measured on Magpix® and coagulation using Stago/Rotational Thromboelastometry.
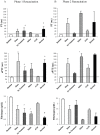
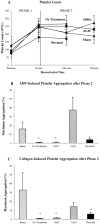
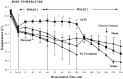
Results: After Phase 1, saline-controls, no-treatment and Hextend® groups showed significant falls in white and red cells, hemoglobin and hematocrit (up to 30%), whereas ALM animals had similar values to shams (9-15% losses). After Phase 2, these deficits in non-ALM groups were accompanied by profound systemic inflammation. In contrast, after Phase 1 ALM-treated animals had undetectable plasma levels of IL-1α and IL-1β, and IL-2, IL-6 and TNF-α were below baseline, and after Phase 2 they were less or similar to shams. Non-ALM groups (except shams) also lost their ability to aggregate platelets, had lower plasma fibrinogen levels, and were hypocoagulable. ALM-treated animals had 50-fold higher ADP-induced platelet aggregation, and 9.3-times higher collagen-induced aggregation compared to saline-controls, and had little or no coagulopathy with significantly higher fibrinogen shifting towards baseline. Hextend® had poor outcomes.
Conclusions: Small-volume ALM bolus/drip mounted a frontline defense against non-compressible traumatic hemorrhage by defending immune cell numbers, suppressing systemic inflammation, improving platelet aggregation and correcting coagulopathy. Saline-controls were equivalent to no-treatment. Possible mechanisms of ALM's immune-bolstering effect are discussed.
Conflict of interest statement
Figures
Similar articles
-
3% NaCl adenosine, lidocaine, Mg2+ (ALM) bolus and 4 hours "drip" infusion reduces noncompressible hemorrhage by 60% in a rat model.J Trauma Acute Care Surg. 2017 Jun;82(6):1063-1072. doi: 10.1097/TA.0000000000001454. J Trauma Acute Care Surg. 2017. PMID: 28520687
-
Adenosine, lidocaine and Mg2+ (ALM) induces a reversible hypotensive state, reduces lung edema and prevents coagulopathy in the rat model of polymicrobial sepsis.J Trauma Acute Care Surg. 2014 Sep;77(3):471-8. doi: 10.1097/TA.0000000000000361. J Trauma Acute Care Surg. 2014. PMID: 25159253
-
Adenosine, lidocaine, and Mg2+ (ALM) resuscitation fluid protects against experimental traumatic brain injury.J Trauma Acute Care Surg. 2018 Jun;84(6):908-916. doi: 10.1097/TA.0000000000001874. J Trauma Acute Care Surg. 2018. PMID: 29554045
-
Adenosine, lidocaine, and Mg2+ (ALM): From cardiac surgery to combat casualty care--Teaching old drugs new tricks.J Trauma Acute Care Surg. 2016 Jan;80(1):135-45. doi: 10.1097/TA.0000000000000881. J Trauma Acute Care Surg. 2016. PMID: 26683400 Review.
-
Mechanistic considerations for adenosine-lidocaine-magnesium (ALM) in controlling coagulopathy.Trends Pharmacol Sci. 2023 Jun;44(6):324-334. doi: 10.1016/j.tips.2023.01.006. Epub 2023 Feb 18. Trends Pharmacol Sci. 2023. PMID: 36805364 Review.
Cited by
-
Conventional and Specific-Pathogen Free Rats Respond Differently to Anesthesia and Surgical Trauma.Sci Rep. 2019 Jun 28;9(1):9399. doi: 10.1038/s41598-019-45871-z. Sci Rep. 2019. PMID: 31253875 Free PMC article.
-
Living in a Hostile World: Inflammation, New Drug Development, and Coronavirus.Front Immunol. 2021 Jan 22;11:610131. doi: 10.3389/fimmu.2020.610131. eCollection 2020. Front Immunol. 2021. PMID: 33552070 Free PMC article. Review.
-
Platelet dysfunction after trauma: From mechanisms to targeted treatment.Transfusion. 2022 Aug;62 Suppl 1(Suppl 1):S281-S300. doi: 10.1111/trf.16971. Epub 2022 Jun 24. Transfusion. 2022. PMID: 35748694 Free PMC article. Review. No abstract available.
-
Antiallodynic and anti-inflammatory effects of intrathecal R-PIA in a rat model of vincristine-induced peripheral neuropathy.Korean J Anesthesiol. 2020 Oct;73(5):434-444. doi: 10.4097/kja.19481. Epub 2020 Feb 12. Korean J Anesthesiol. 2020. PMID: 32046474 Free PMC article.
-
Fluids of the Future.Front Vet Sci. 2021 Jan 21;7:623227. doi: 10.3389/fvets.2020.623227. eCollection 2020. Front Vet Sci. 2021. PMID: 33553287 Free PMC article. Review.
References
-
- Stannard A, Morrison JJ, Scott DJ, Ivatury RA, Ross JD, Rasmussen TE. The epidemiology of noncompressible torso hemorrhage in the wars in Iraq and Afghanistan. J Trauma Acute Care Surg 2013. 74(3):830–4. doi: 10.1097/TA.0b013e31827a3704 - DOI - PubMed
-
- Kisat M, Morrison JJ, Hashmi ZG, Efron DT, Rasmussen TE, Haider AH. Epidemiology and outcomes of non-compressible torso hemorrhage. J Surg Res. 2013;184(1):414–21. doi: 10.1016/j.jss.2013.05.099 - DOI - PubMed
-
- Eastridge BJ, Mabry RL, Seguin P, Cantrell J, Tops T, Uribe P, et al. Death on the battlefield (2001–2011): implications for the future of combat casualty care. J Trauma Acute Care Surg. 2012;73(6 Suppl 5):S431–7. doi: 10.1097/TA.0b013e3182755dcc - DOI - PubMed
-
- Butler FK, Holcomb JB, Schreiber MA, Kotwal RS, Jenkins DA, Champion HR, et al. Fluid Resuscitation for Hemorrhagic Shock in Tactical Combat Casualty Care: TCCC Guidelines Change 14–01–2 June 2014. J Spec Oper Med 2014;14(3):13–28. - PubMed
-
- Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60(6 (Suppl)):S3–11. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

