Sequential inflammatory processes define human progression from M. tuberculosis infection to tuberculosis disease
- PMID: 29145483
- PMCID: PMC5689825
- DOI: 10.1371/journal.ppat.1006687
Sequential inflammatory processes define human progression from M. tuberculosis infection to tuberculosis disease
Abstract
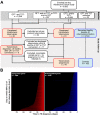
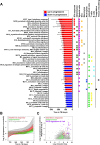
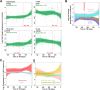
Our understanding of mechanisms underlying progression from Mycobacterium tuberculosis infection to pulmonary tuberculosis disease in humans remains limited. To define such mechanisms, we followed M. tuberculosis-infected adolescents longitudinally. Blood samples from forty-four adolescents who ultimately developed tuberculosis disease (“progressors”) were compared with those from 106 matched controls, who remained healthy during two years of follow up. We performed longitudinal whole blood transcriptomic analyses by RNA sequencing and plasma proteome analyses using multiplexed slow off-rate modified DNA aptamers. Tuberculosis progression was associated with sequential modulation of immunological processes. Type I/II interferon signalling and complement cascade were elevated 18 months before tuberculosis disease diagnosis, while changes in myeloid inflammation, lymphoid, monocyte and neutrophil gene modules occurred more proximally to tuberculosis disease. Analysis of gene expression in purified T cells also revealed early suppression of Th17 responses in progressors, relative to M. tuberculosis-infected controls. This was confirmed in an independent adult cohort who received BCG re-vaccination; transcript expression of interferon response genes in blood prior to BCG administration was associated with suppression of IL-17 expression by BCG-specific CD4 T cells 3 weeks post-vaccination. Our findings provide a timeline to the different immunological stages of disease progression which comprise sequential inflammatory dynamics and immune alterations that precede disease manifestations and diagnosis of tuberculosis disease. These findings have important implications for developing diagnostics, vaccination and host-directed therapies for tuberculosis.
Trial registration: Clincialtrials.gov, NCT01119521.
Conflict of interest statement
TJS, APN, EGT, AA, WAH, and DEZ are co-inventors on a patent of the 16-gene transcriptomic correlate of risk of TB. DS, MAdG, TH and UAO are current or former employees of or hold stock options in SomaLogic, Inc. and received funding from the Bill & Melinda Gates Foundation (OPP1091720). This does not alter our adherence to all PLOS Pathogens policies on sharing data and materials. All other authors have declared that no competing interests exist.
Figures


References
-
- Houben RMGJ, Dodd PJ. The Global Burden of Latent Tuberculosis Infection: A Re-estimation Using Mathematical Modelling. PLoS Med. 2016;13: e1002152 doi: 10.1371/journal.pmed.1002152 - DOI - PMC - PubMed
-
- Thwaites GE, Nguyen DB, Nguyen HD, Hoang TQ, Do TTO, Nguyen TCT, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004;351: 1741–1751. doi: 10.1056/NEJMoa040573 - DOI - PubMed
-
- Tobin DM, Roca FJ, Oh SF, McFarland R, Vickery TW, Ray JP, et al. Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell. 2012;148: 434–446. doi: 10.1016/j.cell.2011.12.023 - DOI - PMC - PubMed
-
- Wilkinson KA, Wilkinson RJ, Pathan A, Ewer K, Prakash M, Klenerman P, et al. Ex vivo characterization of early secretory antigenic target 6-specific T cells at sites of active disease in pleural tuberculosis. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2005;40: 184–187. doi: 10.1086/426139 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

