Early erythropoiesis-stimulating agents in preterm or low birth weight infants
- PMID: 29145693
- PMCID: PMC6486170
- DOI: 10.1002/14651858.CD004863.pub5
Early erythropoiesis-stimulating agents in preterm or low birth weight infants
Update in
-
Early erythropoiesis-stimulating agents in preterm or low birth weight infants.Cochrane Database Syst Rev. 2020 Feb 11;2(2):CD004863. doi: 10.1002/14651858.CD004863.pub6. Cochrane Database Syst Rev. 2020. PMID: 32048730 Free PMC article.
Abstract
Background: Preterm infants have low plasma levels of erythropoietin (EPO), providing a rationale for the use of erythropoiesis-stimulating agents (ESAs) to prevent or treat anaemia and to provide neuro protection and protection against necrotising enterocolitis (NEC). Darbepoetin (Darbe) and EPO are currently available ESAs.
Objectives: To assess the effectiveness and safety of ESAs (erythropoietin (EPO) and/or Darbe) initiated early (before eight days after birth) compared with placebo or no intervention in reducing red blood cell (RBC) transfusions, adverse neurological outcomes, and feeding intolerance including necrotising enterocolitis (NEC) in preterm and/or low birth weight infants. Primary objective for studies that primarily investigate the effectiveness and safety of ESAs administered early in reducing red blood cell transfusions:To assess the effectiveness and safety of ESAs initiated early in reducing red blood cell transfusions in preterm infants. Secondary objectives:Review authors performed subgroup analyses of low (≤ 500 IU/kg/week) and high (> 500 IU/kg/week) doses of EPO and the amount of iron supplementation provided: none, low (≤ 5 mg/kg/d), and high (> 5 mg/kg/d). Primary objective for studies that primarily investigate the neuro protective effectiveness of ESAs:To assess the effectiveness and safety of ESAs initiated early in reducing adverse neurological outcomes in preterm infants. Primary objective for studies that primarily investigate the effectiveness of EPO or Darbe administered early in reducing feeding intolerance:To assess the effectiveness and safety of ESAs administered early in reducing feeding intolerance (and NEC) in preterm infants. Other secondary objectives:To compare the effectiveness of ESAs in reducing the incidence of adverse events and improving long-term neurodevelopmental outcomes.
Search methods: We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 2), MEDLINE via PubMed (1966 to 10 March 2017), Embase (1980 to 10 March 2017), and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to 10 March 2017). We searched clinical trials databases, conference proceedings, and reference lists of retrieved articles for randomised and quasi-randomised controlled trials.
Selection criteria: Randomised and quasi-randomised controlled trials of early initiation of EAS treatment versus placebo or no intervention in preterm or low birth weight infants.
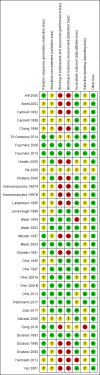
Data collection and analysis: We used the methods described in the Cochrane Handbook for Systematic Reviews of Interventions and the GRADE approach to assess the quality of evidence.
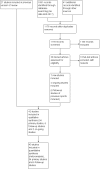
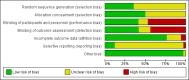
Main results: This updated review includes 34 studies enrolling 3643 infants. All analyses compared ESAs versus a control consisting of placebo or no treatment.Early ESAs reduced the risk of 'use of one or more [red blood cell] RBC transfusions' (typical risk ratio (RR) 0.79, 95% confidence interval (CI) 0.74 to 0.85; typical risk difference (RD) -0.14, 95% CI -0.18 to -0.10; I2 = 69% for RR and 62% for RD (moderate heterogeneity); number needed to treat for an additional beneficial outcome (NNTB) 7, 95% CI 6 to 10; 19 studies, 1750 infants). The quality of the evidence was low.Necrotising enterocolitis was significantly reduced in the ESA group compared with the placebo group (typical RR 0.69, 95% CI 0.52 to 0.91; typical RD -0.03, 95% CI -0.05 to -0.01; I2 = 0% for RR and 22% for RD (low heterogeneity); NNTB 33, 95% CI 20 to 100; 15 studies, 2639 infants). The quality of the evidence was moderate.Data show a reduction in 'Any neurodevelopmental impairment at 18 to 22 months' corrected age in the ESA group (typical RR 0.62, 95% CI 0.48 to 0.80; typical RD -0.08, 95% CI -0.12 to -0.04; NNTB 13, 95% CI 8 to 25. I2 = 76% for RR (high heterogeneity) and 66% for RD (moderate); 4 studies, 1130 infants). The quality of the evidence was low.Results reveal increased scores on the Bayley-II Mental Development Index (MDI) at 18 to 24 months in the ESA group (weighted mean difference (WMD) 8.22, 95% CI 6.52 to 9.92; I2 = 97% (high heterogeneity); 3 studies, 981 children). The quality of the evidence was low.The total volume of RBCs transfused per infant was reduced by 7 mL/kg. The number of RBC transfusions per infant was minimally reduced, but the number of donors to whom infants who were transfused were exposed was not significantly reduced. Data show no significant difference in risk of stage ≥ 3 retinopathy of prematurity (ROP) with early EPO (typical RR 1.24, 95% CI 0.81 to 1.90; typical RD 0.01, 95% CI -0.02 to 0.04; I2 = 0% (no heterogeneity) for RR; I2 = 34% (low heterogeneity) for RD; 8 studies, 1283 infants). Mortality was not affected, but results show significant reductions in the incidence of intraventricular haemorrhage (IVH) and periventricular leukomalacia (PVL).
Authors' conclusions: Early administration of ESAs reduces the use of red blood cell (RBC) transfusions, the volume of RBCs transfused, and donor exposure after study entry. Small reductions are likely to be of limited clinical importance. Donor exposure probably is not avoided, given that all but one study included infants who had received RBC transfusions before trial entry. This update found no significant difference in the rate of ROP (stage ≥ 3) for studies that initiated EPO treatment at less than eight days of age, which has been a topic of concern in earlier versions of this review. Early EPO treatment significantly decreased rates of IVH, PVL, and NEC. Neurodevelopmental outcomes at 18 to 22 months and later varied in published studies. Ongoing research should evaluate current clinical practices that will limit donor exposure. Promising but conflicting results related to the neuro protective effect of early EPO require further study. Very different results from the two largest published trials and high heterogeneity in the analyses indicate that we should wait for the results of two ongoing large trials before drawing firm conclusions. Administration of EPO is not currently recommended because limited benefits have been identified to date. Use of darepoetin requires further study.
Conflict of interest statement
None.
Figures







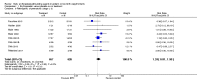



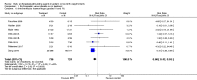





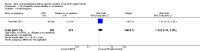

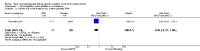

































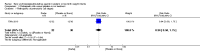






































Update of
-
Early erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants.Cochrane Database Syst Rev. 2014 Apr 26;(4):CD004863. doi: 10.1002/14651858.CD004863.pub4. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2017 Nov 16;11:CD004863. doi: 10.1002/14651858.CD004863.pub5. PMID: 24771408 Updated.
References
References to studies included in this review
-
- Avent M, Cory BJ, Galpin J, Ballot DE, Cooper PA, Sherman G, et al. A comparison of high versus low dose recombinant human erythropoietin versus blood transfusion in the management of anaemia of prematurity in a developing country. Journal of Tropical Pediatrics 2002;48(4):227‐33. [PUBMED: 12200985] - PubMed
-
- Carnielli V, Montini G, Riol R, Dall'Amico R, Cantarutti F. Effect of high doses of human recombinant erythropoietin on the need for blood transfusions in preterm infants. Journal of Pediatrics 1992;121(1):98‐102. [PUBMED: 1625101] - PubMed
-
- Chang L, Liu W, Liao C, Zhao X. Preventive effects of different dosages of recombinant human erythropoietin on anemia of premature infants. Journal of Tongji Medical University/Tong Ji Yi Ke da Xue Xue Bao 1998;18(4):239‐42. [PUBMED: 10806855] - PubMed
References to studies excluded from this review
-
- Al Mofada SM. Safety and efficacy of early erythropoietin administration to pre‐term infants: a preliminary report. Medical Science Research 1994;22(10):749‐50. [EMBASE: 1994345936]
-
- Amin AA, Alzahrani DM. Efficacy of erythropoietin in premature infants. Saudi Medical Journal 2002;23(3):287‐90. [PUBMED: 11938417] - PubMed
-
- Basiri B, Shokouhi M, Pezeshki N, Torabian S. Beneficial erythropoietic effects of recombinant human erythropoietin in very low‐birth weight infants: a single‐center randomized double‐blinded placebo‐controlled trial. Journal of Clinical Neonatology 2015;4(2):87‐90. [EMBASE: 2015952409]
-
- Brown MS, Keith JF 3rd. Comparison between two and five doses a week of recombinant erythropoietin for anemia of prematurity: a randomized trial. Pediatrics 1999;104(2 Pt 1):210‐5. [PUBMED: 10428996] - PubMed
References to ongoing studies
-
- NCT01378273. Preterm Erythropoietin Neuroprotection Trial (PENUT Trial). clinicaltrials.gov/show/NCT01378273 (first received 20 June 2011).
-
- NCT02550054. Erythropoietin in Premature Infants to Prevent Encephalopathy [Erythropoietin in Premature Infants to Prevent Encephalopathy: A Multi‐centre Randomized Blinded Controlled Study of the Efficacy of Erythropoietin in China]. clinicaltrials.gov/show/NCT02550054 (first received 04 September 2015).
Additional references
-
- Baer VL, Lambert DK, Henry E, Snow GL, Christensen RD. Red blood cell transfusion of preterm neonates with grade 1 intraventricular hemorrhage is associated with extension to a grade 3 or 4 hemorrhage. Transfusion 2011;51(9):1933‐9. [DOI: 10.1111/j.1537-2995.2011.03081.x; PUBMED: 21382049] - DOI - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

