Testosterone replacement in transgenic sickle cell mice controls priapic activity and upregulates PDE5 expression and eNOS activity in the penis
- PMID: 29145710
- PMCID: PMC5745275
- DOI: 10.1111/andr.12442
Testosterone replacement in transgenic sickle cell mice controls priapic activity and upregulates PDE5 expression and eNOS activity in the penis
Abstract
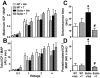
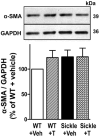
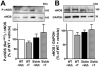
Sickle cell disease (SCD)-associated priapism is characterized by decreased nitric oxide (NO) signaling and downregulated phosphodiesterase (PDE)5 protein expression and activity in the penis. Priapism is also associated with testosterone deficiency, but molecular mechanisms underlying testosterone effects in the penis in SCD are not known. Given the critical role of androgens in erection physiology and NO synthase (NOS)/PDE5 expression, we hypothesized that testosterone replacement to eugonadal testosterone levels reduces priapism by reversing impaired endothelial (e)NOS activity and molecular abnormalities involving PDE5. Adult male transgenic Berkeley sickle cell (Sickle) and wild-type (WT) mice were implanted with testosterone pellets, which release 1.2 μg testosterone/day for 21 days, or vehicle. After 21 days, animals underwent erectile function assessment followed by collection of blood for serum testosterone measurements, penes for molecular analysis, and seminal vesicles as testosterone-responsive tissue. Serum testosterone levels were measured by radioimmunoassay; protein expressions of PDE5, α-smooth muscle actin, eNOS and nNOS, and phosphorylation of PDE5 at Ser-92, eNOS at Ser-1177, neuronal (n) NOS at Ser-1412, and Akt at Ser-473 were measured by Western blot in penile tissue. Testosterone treatment reversed downregulated serum testosterone levels and increased (p < 0.05) the weight of seminal vesicles in Sickle mice to levels comparable to that of WT mice, indicating restored testosterone levels in Sickle mice. Testosterone treatment reduced (p < 0.05) prolonged detumescence in Sickle mice and normalized downregulated P-PDE5 (Ser-92), PDE5, P-eNOS (Ser-1177), and P-Akt (Ser-473) protein expressions in the Sickle mouse penis. Testosterone treatment did not affect P-nNOS (Ser-1412), eNOS, nNOS, or α-smooth muscle actin protein expressions in the Sickle mouse penis. In conclusion, in the mouse model of human SCD, increasing testosterone to eugonadal levels reduced priapic activity and reversed impaired Akt/eNOS activity and PDE5 protein expression in the penis.
Keywords: Akt; eNOS phosphorylation; nNOS phosphorylation.
© 2017 American Society of Andrology and European Academy of Andrology.
Figures
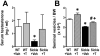

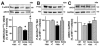
Similar articles
-
Sildenafil promotes eNOS activation and inhibits NADPH oxidase in the transgenic sickle cell mouse penis.J Sex Med. 2014 Feb;11(2):424-30. doi: 10.1111/jsm.12391. Epub 2013 Nov 20. J Sex Med. 2014. PMID: 24251665 Free PMC article.
-
Subacute Hemolysis in Sickle Cell Mice Causes Priapism Secondary to NO Imbalance and PDE5 Dysregulation.J Sex Med. 2015 Sep;12(9):1878-85. doi: 10.1111/jsm.12976. Epub 2015 Sep 7. J Sex Med. 2015. PMID: 26346631 Free PMC article.
-
Post-translational inactivation of endothelial nitric oxide synthase in the transgenic sickle cell mouse penis.J Sex Med. 2011 Feb;8(2):419-26. doi: 10.1111/j.1743-6109.2010.02123.x. Epub 2010 Dec 8. J Sex Med. 2011. PMID: 21143412 Free PMC article.
-
Nitric Oxide Resistance in Priapism Associated with Sickle Cell Disease: Mechanisms, Therapeutic Challenges, and Future Directions.J Pharmacol Exp Ther. 2024 Jul 18;390(2):203-212. doi: 10.1124/jpet.123.001962. J Pharmacol Exp Ther. 2024. PMID: 38262744 Review.
-
Phosphodiesterase type 5 regulation in the penile corpora cavernosa.J Sex Med. 2009 Mar;6 Suppl 3:203-9. doi: 10.1111/j.1743-6109.2008.01179.x. J Sex Med. 2009. PMID: 19267844 Review.
Cited by
-
Soluble guanylate cyclase stimulators and activators: new horizons in the treatment of priapism associated with sickle cell disease.Front Pharmacol. 2024 Feb 7;15:1357176. doi: 10.3389/fphar.2024.1357176. eCollection 2024. Front Pharmacol. 2024. PMID: 38384294 Free PMC article. Review.
-
The effect of red blood cell disorders on male fertility and reproductive health.Nat Rev Urol. 2024 May;21(5):303-316. doi: 10.1038/s41585-023-00838-8. Epub 2024 Jan 3. Nat Rev Urol. 2024. PMID: 38172196 Review.
-
Hydroxyurea does not reverse functional alterations of the nitric oxide-cGMP pathway associated with priapism phenotype in corpus cavernosum from sickle cell mouse.PLoS One. 2023 Oct 9;18(10):e0292706. doi: 10.1371/journal.pone.0292706. eCollection 2023. PLoS One. 2023. PMID: 37812620 Free PMC article.
-
Resolution of Acute Priapism in Two Children With Sickle Cell Disease Who Received Nitrous Oxide.Acad Emerg Med. 2019 Sep;26(9):1102-1105. doi: 10.1111/acem.13822. Epub 2019 Aug 1. Acad Emerg Med. 2019. PMID: 31228879 Free PMC article.
-
What is the effectiveness of surgical and non-surgical therapies in the treatment of ischemic priapism in patients with sickle cell disease? A systematic review by the EAU Sexual and Reproductive Health Guidelines Panel.Int J Impot Res. 2024 Feb;36(1):20-35. doi: 10.1038/s41443-022-00590-4. Epub 2022 Aug 8. Int J Impot Res. 2024. PMID: 35941221
References
-
- Abbasi AA, Prasad AS, Ortega J, Congco E, Oberleas D. Gonadal function abnormalities in sickle cell anemia. Studies in adult male patients. Ann Intern Med. 1976;85:601–605. - PubMed
-
- Bautista Niño PK, Durik M, Danser AH, de Vries R, Musterd-Bhaggoe UM, Meima ME, Kavousi M, Ghanbari M, Hoeijmakers JH, O'Donnell CJ, Franceschini N, Janssen GM, De Mey JG, Liu Y, Shanahan CM, Franco OH, Dehghan A, Roks AJ. Phosphodiesterase 1 regulation is a key mechanism in vascular aging. Clin Sci (Lond) 2015;129:1061–1075. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

