Effectiveness of current and future regimens for treating genotype 3 hepatitis C virus infection: a large-scale systematic review
- PMID: 29145802
- PMCID: PMC5691805
- DOI: 10.1186/s12879-017-2820-z
Effectiveness of current and future regimens for treating genotype 3 hepatitis C virus infection: a large-scale systematic review
Abstract
Background: Six distinct genetic variants (genotypes 1 - 6) of hepatitis C virus (HCV) exist globally. Certain genotypes are more prevalent in particular countries or regions than in others but, globally, genotype 3 (GT3) is the second most common. Patients infected with HCV GT1, 2, 4, 5 or 6 recover to a greater extent, as measured by sustained virological response (SVR), following treatment with regimens based on direct-acting antivirals (DAAs) than after treatment with older regimens based on pegylated interferon (Peg-IFN). GT3, however, is regarded as being more difficult to treat as it is a relatively aggressive genotype, associated with greater liver damage and cancer risk; some subgroups of patients with GT3 infection are less responsive to current licensed DAA treatments. Newer DAAs have become available or are in development.
Methods: According to PRISMA guidance, we conducted a systematic review (and descriptive statistical analysis) of data in the public domain from relevant clinical trial or observational (real-world) study publications within a 5-year period (February 2011 to May 2016) identified by PubMed, Medline In-Process, and Embase searches. This was supplemented with a search of five non-indexed literature sources, comprising annual conferences of the AASLD, APASL, CROI, EASL, and WHO, restricted to a 1-year period (April 2015 to May 2016).
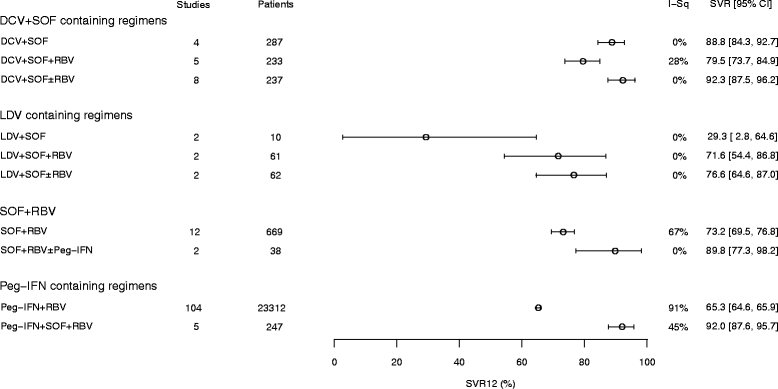
Results: Of the all-oral regimens, the efficacy (SVR12 ≥ 90%) of sofosbuvir plus daclatasvir- and velpatasvir-based regimens in clinical trials supports and reinforces their recommendation by guidelines. Other promising regimens comprise grazoprevir + elbasvir + sofosbuvir, and ombitasvir + paritaprevir/ribavirin + sofosbuvir. Newer regimens incorporating pibrentasvir + glecaprevir or grazoprevir + ruzasvir + MK-3682 (uprifosbuvir), offer all-oral, ribavirin-free SVR12 rates consistently greater than 95%. Observational studies report slightly lower overall SVR rates but reflect corresponding clinical trial data in terms of treatments most likely to achieve good responses.
Conclusions: On the basis of SVR12, we established that for treating GT3 infections (i) regimens incorporating newer DAAs are more effective than those comprising older DAAs, and (ii) ribavirin may be of less benefit in newer DAA regimens than in older DAA regimens. The analysis provides evidence that DAA regimens can replace Peg-IFN-based regimens for GT3 infection.
Keywords: Cirrhosis; Co-infection; Direct-acting antiviral; Genotype 3; Hepatitis C virus; Systematic literature review.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable; manuscript is a systematic review of published data.
Consent for publication
Not applicable; manuscript is a systematic review of published data.
Competing interests
Hosnieh Fathi is an employee of Almirall Ltd. (formerly of Bristol-Myers Squibb Pharmaceuticals Ltd), and has no conflicts of interest to declare.
Andrew Clark is an employee of Bristol-Myers Squibb Pharmaceuticals Ltd., and has no conflicts of interest to declare.
Nathan Hill is an employee of Bristol-Myers Squibb Pharmaceuticals Ltd., and has no conflicts of interest to declare.
Geoffrey Dusheiko has acted as an advisor to Bristol-Myers Squibb, Gilead, Merck, Janssen, and Abbvie.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2014. Available from: http://www.easl.eu/medias/EASLimg/News/easl_recommendations_hcv_2014_ful.... Accessed Jan 2017. - PubMed
-
- National Institute for Health and Care Excellence. Interferon alfa (pegylated and non-pegylated) and ribavirin for the treatment of chronic hepatitis C. NICE technology appraisal guidance [TA75]. 2004. 22 August 2016. Available from: https://www.nice.org.uk/guidance/ta75. Accessed Jan 2017.
-
- National Institute for Health and Care Excellence. Peginterferon alfa and ribavirin for the treatment of chronic hepatitis C. NICE technology appraisal guidance [TA200]. 2010. 15 August 2014. Available from: https://www.nice.org.uk/guidance/TA200. Accessed Jan 2017.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

