COPD online-rehabilitation versus conventional COPD rehabilitation - rationale and design for a multicenter randomized controlled trial study protocol (CORe trial)
- PMID: 29145831
- PMCID: PMC5689178
- DOI: 10.1186/s12890-017-0488-1
COPD online-rehabilitation versus conventional COPD rehabilitation - rationale and design for a multicenter randomized controlled trial study protocol (CORe trial)
Abstract
Background: Rehabilitation of patients with chronic obstructive pulmonary disease (COPD) is a key treatment in COPD. However, despite the existing evidence and a strong recommendation from lung associations worldwide, 50% of patients with COPD decline to participate in COPD rehabilitation program and 30-50% drop-out before completion. The main reasons are severe symptoms, inflexible accessibility and necessity for transportation. Currently there are no well-established and evident rehabilitation alternatives. Supervised online screen rehabilitation could be a useful approach to increase accessibility and compliance. The aim of this multicenter RCT study is to compare the potential benefits of a 10-week online COPD rehabilitation program (CORe) with conventional outpatient COPD rehabilitation (CCRe).
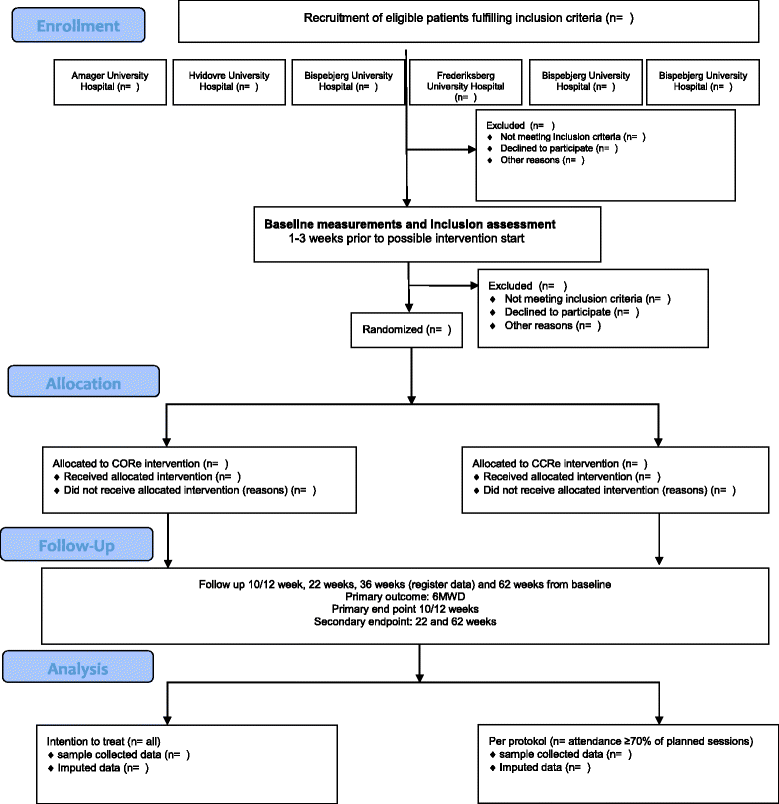
Methods: This study is a randomized assessor- and statistician blinded superiority multicenter trial with two parallel groups, employing 1:1 allocation to the intervention and the comparison group.On the basis of a sample size calculation, 134 patients with severe or very severe COPD and eligible to conventional hospital based outpatient COPD rehabilitation will be included and randomized from eight different hospitals. The CORe intervention group receives group supervised resistance- and endurance training and patient education, 60 min, three times/week for 10 weeks at home via online-screen. The CCRe comparison group receives group based supervised resistance- and endurance training and patient education, 90 min, two times/week for 10 weeks (two hospitals) or 12 weeks (six hospitals) in groups at the local hospital. The primary outcome is change in the 6-min walking distance after 10/12 weeks; the secondary outcomes are changes in 30 s sit-to-stand chair test, physical activity level, symptoms, anxiety and depression symptoms, disease specific and generic quality of life. Primary endpoint is 10/12 weeks from baseline, while secondary endpoints are 22, 36, 62 weeks from baseline assessments.
Discussion: The study will likely contribute to knowledge regarding COPD tele-rehabilitation and to which extent it is more feasible and thereby more efficient than conventional COPD rehabilitation in patients with severe and very severe COPD.
Trial registration: Clinicaltrials.gov Identifier: NCT02667171 . Registration data: January 28th 2016.
Keywords: COPD; Exercise; Multicenter; Pulmonary rehabilitation; Quality of life; Randomized controlled trial; Tele-rehabilitation.
Conflict of interest statement
Ethics approval and consent to participate
The trial protocol is approved by the Ethics Committee of the Capital Region of Denmark (H-15019380) and written informed consent was obtained from all patients. The Danish Data Protection Agency approved the research database (j.nr.: 2012–58-0004) and the trial is registered at
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Agusti A, Hurd S, Jones P, Fabbri LM, FVC M, et al. Global initiative for chronic obstructive lung. 2017.
-
- Puhan M, Scharplatz M, Troosters T, Walters EH, Steurer J. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2016;(12). 10.1002/14651858.CD005305.pub2. - PubMed
-
- Spruit MA, Singh SJ, Garvey C, et al. An official American thoracic society/European respiratory society statement: Key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8). doi:10.1164/rccm.201309-1634ST. - PubMed
-
- Uk JB, Woodcock A, Knight A, et al. BTS Guideline on Pulmonary Rehabilitation in Adults. An Int J Respir Med. 2013;68(2):ii1–i31.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical