Are mice good models for human neuromuscular disease? Comparing muscle excursions in walking between mice and humans
- PMID: 29145886
- PMCID: PMC5689180
- DOI: 10.1186/s13395-017-0143-9
Are mice good models for human neuromuscular disease? Comparing muscle excursions in walking between mice and humans
Abstract
Background: The mouse is one of the most widely used animal models to study neuromuscular diseases and test new therapeutic strategies. However, findings from successful pre-clinical studies using mouse models frequently fail to translate to humans due to various factors. Differences in muscle function between the two species could be crucial but often have been overlooked. The purpose of this study was to evaluate and compare muscle excursions in walking between mice and humans.
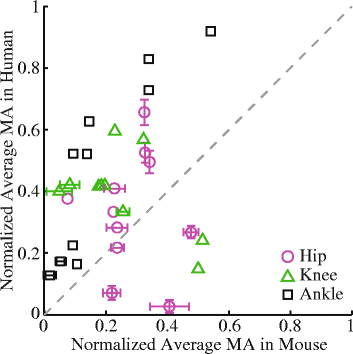
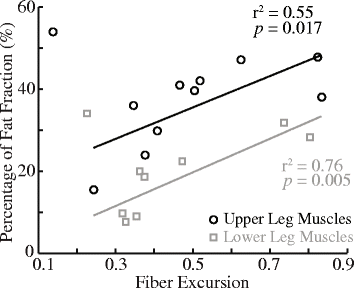
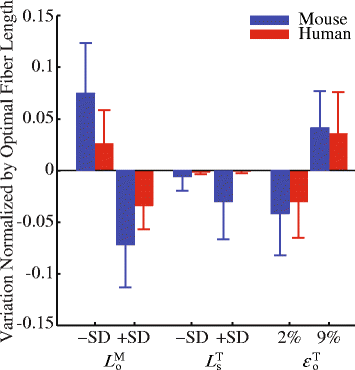
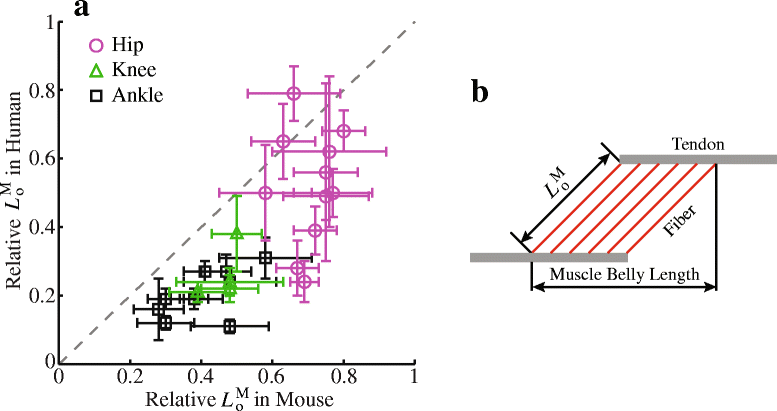
Methods: Recently published musculoskeletal models of the mouse hindlimb and human lower limb were used to simulate muscle-tendon dynamics during mouse and human walking, a key daily activity. Muscle fiber length changes (fiber excursions) of 25 muscle homologs in the two species were calculated from these simulations and then compared. To understand potential causes of differences in fiber excursions in walking, joint excursions and muscle moment arms were also compared across one gait cycle.
Results: Most muscles (19 out of 25 muscles) of the mouse hindlimb had much smaller fiber excursions as compared to human lower limb muscles during walking. For these muscles, fiber excursions in mice were only 48 ± 19% of those in humans. The differences in fiber excursion between the two species were primarily due to the reduced joint excursions and smaller muscle moment arms in mice as compared to humans.
Conclusions: Since progressive neuromuscular diseases, such as Duchenne muscular dystrophy, are known to be accelerated by damage accumulated from active muscle lengthening, these results suggest that biomechanical differences in muscle function during walking between mice and humans may impede the translations of knowledge gained from mouse models to humans. This knowledge would add a fresh perspective on how pre-clinical studies on mice might be better designed to improve translation to human clinical trials.
Keywords: Biomechanics; Duchenne muscular dystrophy; Mice; Muscle fiber excursion; Musculoskeletal simulation; Neuromuscular diseases; Walking gait.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

