Downregulation of MiR-31 stimulates expression of LATS2 via the hippo pathway and promotes epithelial-mesenchymal transition in esophageal squamous cell carcinoma
- PMID: 29145896
- PMCID: PMC5689139
- DOI: 10.1186/s13046-017-0622-1
Downregulation of MiR-31 stimulates expression of LATS2 via the hippo pathway and promotes epithelial-mesenchymal transition in esophageal squamous cell carcinoma
Abstract
Background: Dysregulation of miRNAs is associated with cancer development by coordinately suppressing abundant target genes. Emerging evidence indicates that miR-31 plays a dual role in tumorigenicity. However, whether miR-31 plays as an oncogene in esophageal squamous cell carcinoma (ESCC) and the potential target molecules are still unclear. MiR-31 role in ESCC was investigated and an association of the target molecules with EMT was identified in the progression of ESCC.
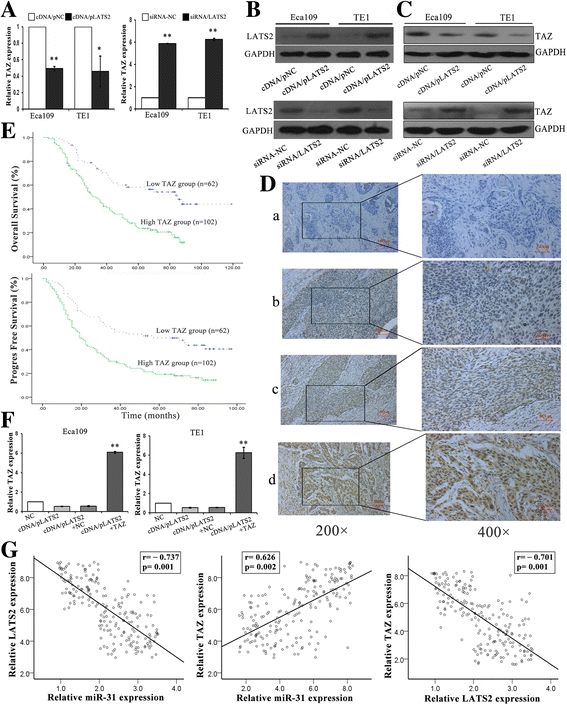
Methods: Western blot assays and qRT-PCR was performed to detect the protein and mRNA levels. We investigated the role of miR-31 in the regulation of LATS2 expression in ESCC cell lines via functional assays both in vivo and in vitro. The luciferase reporter assays was conducted to confirm LATS2 is a potential target of miR-31. Immunohistochemistry was used to measure LATS2 and TAZ expression in normal and ESCC tissue.
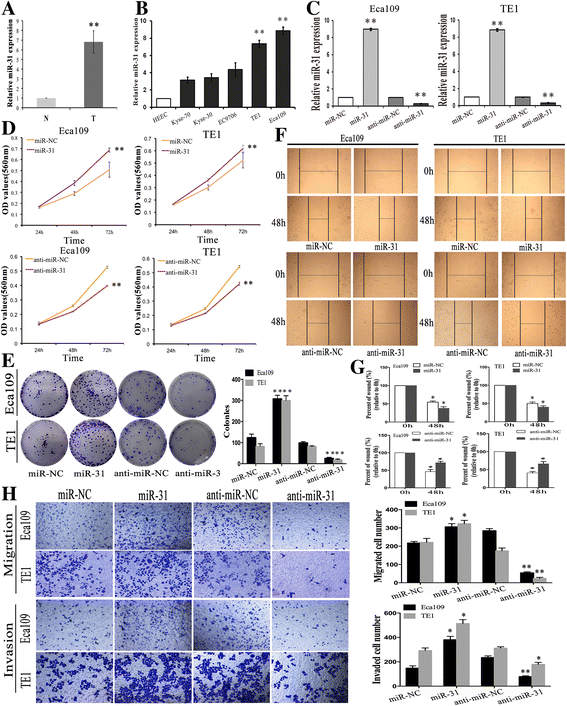
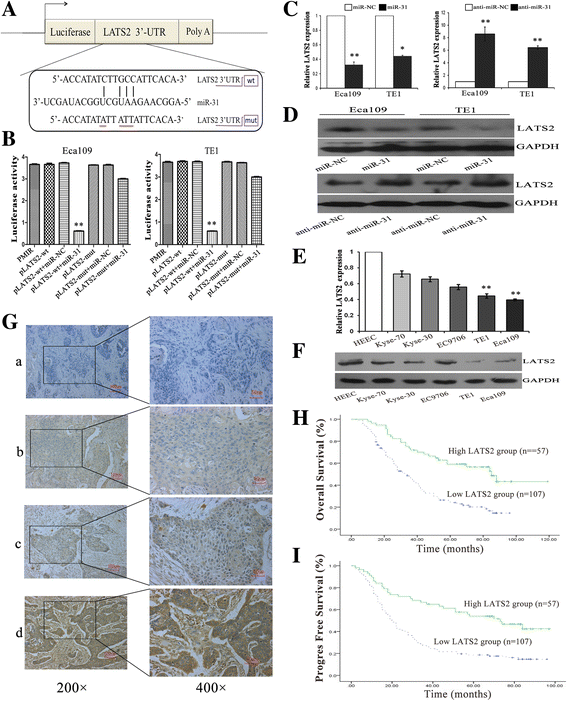
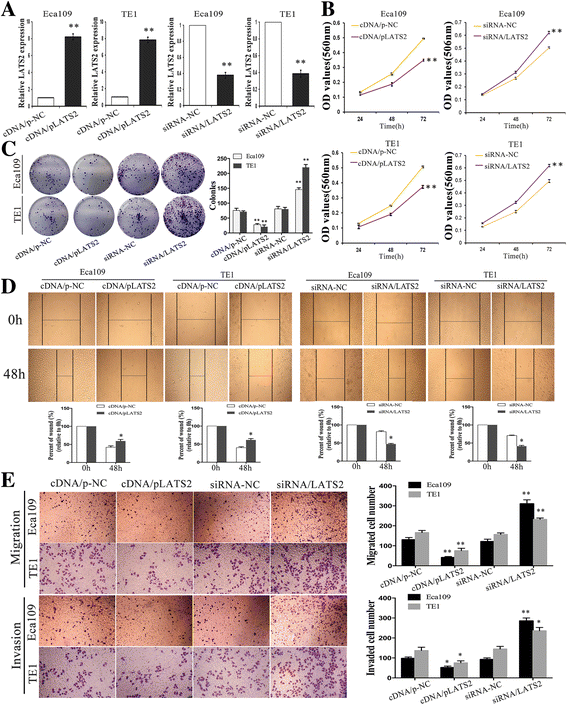
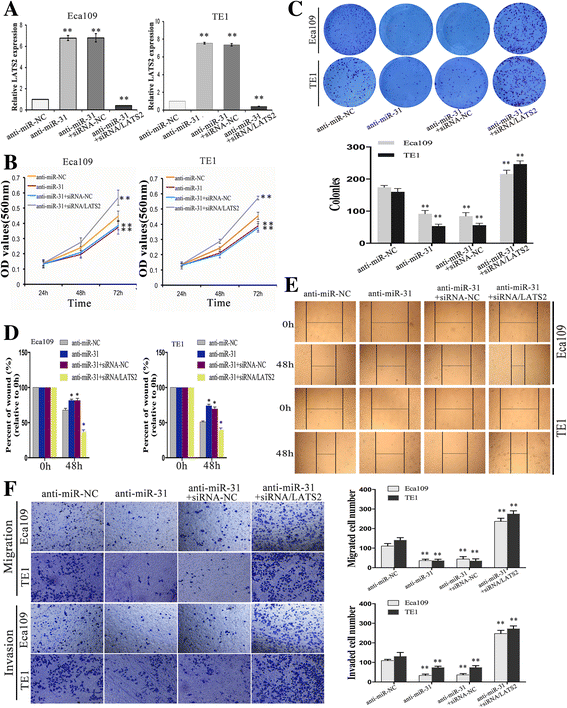
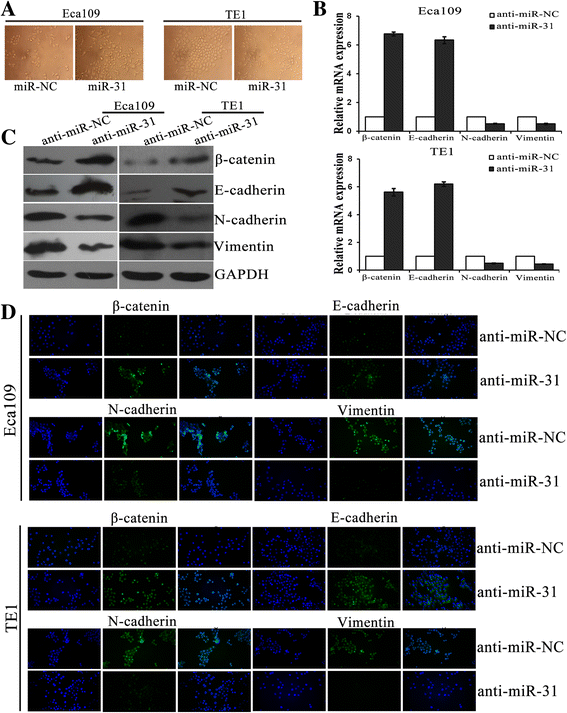
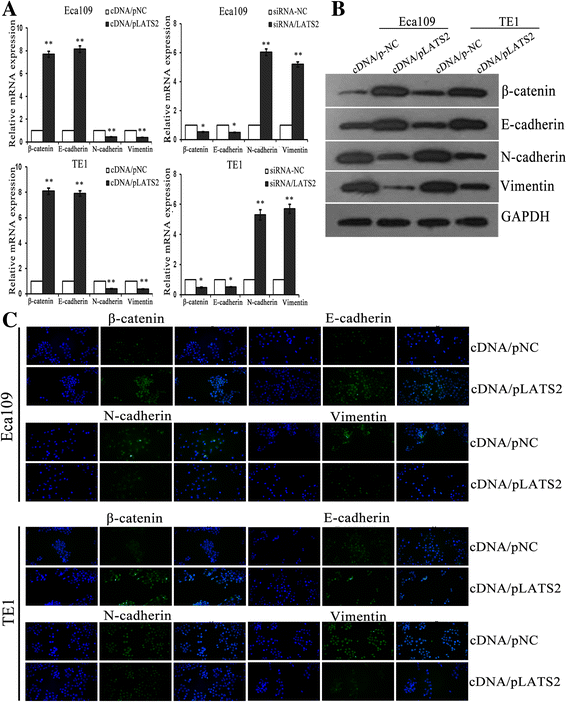
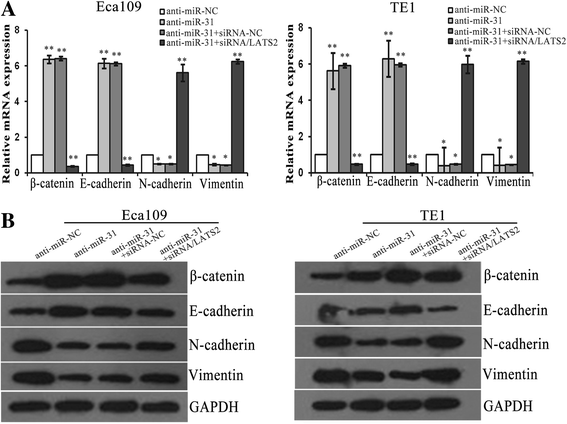
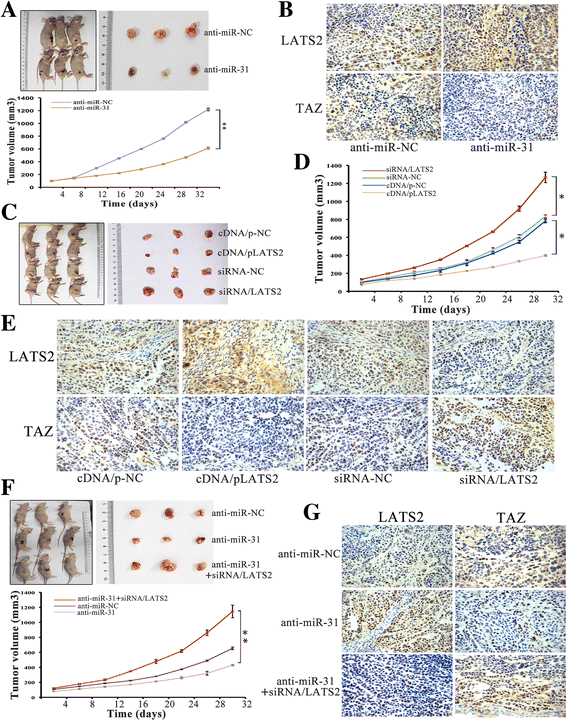
Results: LATS2 is a component of the Hippo tumor-suppressive signaling pathway. Frequent loss of heterozygosity of LATS2 has been reported in esophageal cancer. We analyzed the reciprocal expression regulation of miR-31 and LATS2 and demonstrated that LATS2 expression was elevated by down-regulation of miR-31 at the post-transcriptional level in ESCC. Moreover, miR-31 significantly suppressed the luciferase activity of mRNA combined with the LATS2 3'-UTR, a key molecule in the Hippo pathway. Then, LATS2 consequently promoted the translocation of TAZ, which was examined using immunohistochemistry. Silencing of miR-31 significantly inhibited the cell proliferation, induced apoptosis and decreased the ability of migration/invasion in vitro. LATS2 impedes ESCC cell proliferation and invasion by suppressing miR-31, as well as mice xenograft model in vivo. Meanwhile, the nuclear localization of LATS2 constrained the phosphorylation of TAZ. Then, the expression level of TAZ was notably heightened with a high risk of recurrence compared to that observed in the low-risk patients, as well as, the higher expression associated with a poor survival.
Conclusions: Our study demonstrated that overexpression of miR-31 undertook an oncogenic role in ESCC by repressing expression of LATS2 via the Hippo Pathway and activating epithelial-mesenchymal transition. LATS2 and TAZ could be potential novel molecular markers for predicting the risk of recurrence and prognosis of ESCC.
Keywords: EMT; Esophageal squamous cell carcinoma; Hippo pathway; LATS2; TAZ; miR-31.
Conflict of interest statement
Ethics approval and consent to participate
The research protocol was assessed and approved by the Ethical Committee and Institutional Review Board of the Jinling Hospital, and written informed consent was obtained from each patient included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
MicroRNA-145 Inhibits Cell Migration and Invasion and Regulates Epithelial-Mesenchymal Transition (EMT) by Targeting Connective Tissue Growth Factor (CTGF) in Esophageal Squamous Cell Carcinoma.Med Sci Monit. 2016 Oct 23;22:3925-3934. doi: 10.12659/msm.897663. Med Sci Monit. 2016. PMID: 27771733 Free PMC article.
-
MiR-99a suppresses proliferation, migration and invasion of esophageal squamous cell carcinoma cells through inhibiting the IGF1R signaling pathway.Cancer Biomark. 2017 Dec 6;20(4):527-537. doi: 10.3233/CBM-170345. Cancer Biomark. 2017. PMID: 28800315
-
MiR-130b plays an oncogenic role by repressing PTEN expression in esophageal squamous cell carcinoma cells.BMC Cancer. 2015 Jan 31;15:29. doi: 10.1186/s12885-015-1031-5. BMC Cancer. 2015. PMID: 25637514 Free PMC article.
-
MicroRNAs in esophageal squamous cell carcinoma: Potential biomarkers and therapeutic targets.Cancer Biomark. 2017;19(1):1-9. doi: 10.3233/CBM-160240. Cancer Biomark. 2017. PMID: 28269750 Review.
-
Noncoding RNA Expression Aberration Is Associated with Cancer Progression and Is a Potential Biomarker in Esophageal Squamous Cell Carcinoma.Int J Mol Sci. 2015 Nov 24;16(11):27824-34. doi: 10.3390/ijms161126060. Int J Mol Sci. 2015. PMID: 26610479 Free PMC article. Review.
Cited by
-
Cordycepin Inhibits Growth and Metastasis Formation of MDA-MB-231 Xenografts in Nude Mice by Modulating the Hedgehog Pathway.Int J Mol Sci. 2022 Sep 8;23(18):10362. doi: 10.3390/ijms231810362. Int J Mol Sci. 2022. PMID: 36142286 Free PMC article.
-
Long noncoding RNA MEG3 regulates LATS2 by promoting the ubiquitination of EZH2 and inhibits proliferation and invasion in gallbladder cancer.Cell Death Dis. 2018 Oct 3;9(10):1017. doi: 10.1038/s41419-018-1064-1. Cell Death Dis. 2018. PMID: 30282996 Free PMC article.
-
ESOMIR: a curated database of biomarker genes and miRNAs associated with esophageal cancer.Database (Oxford). 2023 Oct 10;2023:baad063. doi: 10.1093/database/baad063. Database (Oxford). 2023. PMID: 37815872 Free PMC article.
-
The interplay between noncoding RNA and YAP/TAZ signaling in cancers: molecular functions and mechanisms.J Exp Clin Cancer Res. 2022 Jun 14;41(1):202. doi: 10.1186/s13046-022-02403-4. J Exp Clin Cancer Res. 2022. PMID: 35701841 Free PMC article. Review.
-
LATS2 inhibits cell proliferation and metastasis through the Hippo signaling pathway in glioma.Oncol Rep. 2019 May;41(5):2753-2761. doi: 10.3892/or.2019.7065. Epub 2019 Mar 14. Oncol Rep. 2019. PMID: 30896861 Free PMC article.
References
-
- Kamangar F, Qiao YL, Schiller JT, Dawsey SM, Fears T, Sun XD, Abnet CC, Zhao P, Taylor PR, Mark SD. Human papillomavirus serology and the risk of esophageal and gastric cancers: results from a cohort in a high-risk region in China. Int J Cancer. 2006;119(3):579–584. doi: 10.1002/ijc.21871. - DOI - PubMed
-
- He Z, Yi J, Jin L, Pan B, Chen L, Song H. Overexpression of Sirtuin-1 is associated with poor clinical outcome in esophageal squamous cell carcinoma. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2016;37(6):7139–7148. doi: 10.1007/s13277-015-4459-y. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

