Macrophages: Their role, activation and polarization in pulmonary diseases
- PMID: 29146235
- PMCID: PMC7114886
- DOI: 10.1016/j.imbio.2017.11.001
Macrophages: Their role, activation and polarization in pulmonary diseases
Abstract
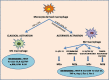
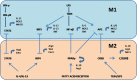
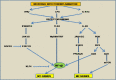
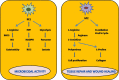
Macrophages, circulating in the blood or concatenated into different organs and tissues constitute the first barrier against any disease. They are foremost controllers of both innate and acquired immunity, healthy tissue homeostasis, vasculogenesis and congenital metabolism. Two hallmarks of macrophages are diversity and plasticity due to which they acquire a wobbling array of phenotypes. These phenotypes are appropriately synchronized responses to a variety of different stimuli from either the tissue microenvironment or - microbes or their products. Based on the phenotype, macrophages are classified into classically activated/(M1) and alternatively activated/(M2) which are further sub-categorized into M2a, M2b, M2c and M2d based upon gene expression profiles. Macrophage phenotype metamorphosis is the regulating factor in initiation, progression, and termination of numerous inflammatory diseases. Several transcriptional factors and other factors controlling gene expression such as miRNAs contribute to the transformation of macrophages at different points in different diseases. Understanding the mechanisms of macrophage polarization and modulation of their phenotypes to adjust to the micro environmental conditions might provide us a great prospective for designing novel therapeutic strategy. In view of the above, this review summarises the activation of macrophages, the factors intricated in activation along with benefaction of macrophage polarization in response to microbial infections, pulmonary toxicity, lung injury and other inflammatory diseases such as chronic obstructive pulmonary dysplasia (COPD), bronchopulmonary dysplasia (BPD), asthma and sepsis, along with the existing efforts to develop therapies targeting this facet of macrophage biology.
Keywords: Alternative activation; Asthma; BPD; COPD; Classical activation; Lung inflammation; M1/M2 macrophages.
Copyright © 2017 Elsevier GmbH. All rights reserved.
Figures

References
-
- Agarwal R., et al. Etiology and outcomes of pulmonary and extrapulmonary acute lung injury/ARDS in a respiratory ICU in North India. Chest. 2006;130(3):724–729. - PubMed
-
- Alam K., et al. Glutathione-redox balance regulates c-rel-driven IL-12 production in macrophages: possible implications in antituberculosis immunotherapy. J. Immunol. 2010;184(6):2918–2929. - PubMed
-
- Amano H., et al. Essential contribution of monocyte chemoattractant protein-1/C-C chemokine ligand-2 to resolution and repair processes in acute bacterial pneumonia. J. Immunol. 2004;172(1):398–409. - PubMed
-
- Anderson C.F., Mosser D.M. A novel phenotype for an activated macrophage: the type 2 activated macrophage. J. Leukoc. Biol. 2002;72(1):101–106. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

