Specific oxygenation of plasma membrane phospholipids by Pseudomonas aeruginosa lipoxygenase induces structural and functional alterations in mammalian cells
- PMID: 29146531
- PMCID: PMC5764228
- DOI: 10.1016/j.bbalip.2017.11.005
Specific oxygenation of plasma membrane phospholipids by Pseudomonas aeruginosa lipoxygenase induces structural and functional alterations in mammalian cells
Erratum in
-
Corrigendum to "Specific oxygenation of plasma membrane phospholipids by Pseudomonas aeruginosa lipoxygenase induces structural and functional alterations in mammalian cells" [BBA-Mol. Cell Biol. Volume 1863/2, Pages 152-164].Biochim Biophys Acta Mol Cell Biol Lipids. 2020 Aug;1865(8):158701. doi: 10.1016/j.bbalip.2020.158701. Epub 2020 Apr 27. Biochim Biophys Acta Mol Cell Biol Lipids. 2020. PMID: 32354679 Free PMC article. No abstract available.
Abstract
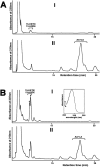
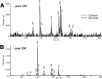
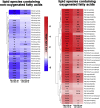
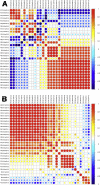
Pseudomonas aeruginosa is a gram-negative pathogen, which causes life-threatening infections in immunocompromized patients. These bacteria express a secreted lipoxygenase (PA-LOX), which oxygenates free arachidonic acid to 15S-hydro(pero)xyeicosatetraenoic acid. It binds phospholipids at its active site and physically interacts with lipid vesicles. When incubated with red blood cells membrane lipids are oxidized and hemolysis is induced but the structures of the oxygenated membrane lipids have not been determined. Using a lipidomic approach, we analyzed the formation of oxidized phospholipids generated during the in vitro incubation of recombinant PA-LOX with human erythrocytes and cultured human lung epithelial cells. Precursor scanning of lipid extracts prepared from these cells followed by multiple reaction monitoring and MS/MS analysis revealed a complex mixture of oxidation products. For human red blood cells this mixture comprised forty different phosphatidylethanolamine and phosphatidylcholine species carrying oxidized fatty acid residues, such as hydroxy-octadecadienoic acids, hydroxy- and keto-eicosatetraenoic acid, hydroxy-docosahexaenoic acid as well as oxygenated derivatives of less frequently occurring polyenoic fatty acids. Similar oxygenation products were also detected when cultured lung epithelial cells were employed but here the amounts of oxygenated lipids were smaller and under identical experimental conditions we did not detect major signs of cell lysis. However, live imaging indicated an impaired capacity for trypan blue exclusion and an augmented mitosis rate. Taken together these data indicate that PA-LOX can oxidize the membrane lipids of eukaryotic cells and that the functional consequences of this reaction strongly depend on the cell type.
Keywords: Biomembranes; Eicosanoids; Fatty acids; Infectious diseases; Lipidomics; Oxidative stress; Phospholipids.
Copyright © 2017 The Authors. Published by Elsevier B.V. All rights reserved.
Figures





Similar articles
-
Secreted lipoxygenase from Pseudomonas aeruginosa exhibits biomembrane oxygenase activity and induces hemolysis in human red blood cells.Arch Biochem Biophys. 2015 Oct 15;584:116-24. doi: 10.1016/j.abb.2015.09.003. Epub 2015 Sep 8. Arch Biochem Biophys. 2015. PMID: 26361973
-
Structural and functional basis of phospholipid oxygenase activity of bacterial lipoxygenase from Pseudomonas aeruginosa.Biochim Biophys Acta. 2016 Nov;1861(11):1681-1692. doi: 10.1016/j.bbalip.2016.08.002. Epub 2016 Aug 5. Biochim Biophys Acta. 2016. PMID: 27500637
-
Oxidative modification of human lipoproteins by lipoxygenases of different positional specificities.J Lipid Res. 1994 Oct;35(10):1749-59. J Lipid Res. 1994. PMID: 7852852
-
The accumulation of free arachidonic acid, diacylglycerols, prostaglandins, and lipoxygenase reaction products in the brain during experimental epilepsy.Adv Neurol. 1986;44:879-902. Adv Neurol. 1986. PMID: 3010683 Review.
-
Redox Epiphospholipidome in Programmed Cell Death Signaling: Catalytic Mechanisms and Regulation.Front Endocrinol (Lausanne). 2021 Feb 19;11:628079. doi: 10.3389/fendo.2020.628079. eCollection 2020. Front Endocrinol (Lausanne). 2021. PMID: 33679610 Free PMC article. Review.
Cited by
-
Pain Control by Targeting Oxidized Phospholipids: Functions, Mechanisms, Perspectives.Front Endocrinol (Lausanne). 2021 Jan 25;11:613868. doi: 10.3389/fendo.2020.613868. eCollection 2020. Front Endocrinol (Lausanne). 2021. PMID: 33569042 Free PMC article. Review.
-
Role of Host and Bacterial Lipids in Pseudomonas aeruginosa Respiratory Infections.Front Immunol. 2022 Jul 4;13:931027. doi: 10.3389/fimmu.2022.931027. eCollection 2022. Front Immunol. 2022. PMID: 35860265 Free PMC article. Review.
-
Pseudomonas aeruginosa utilizes host polyunsaturated phosphatidylethanolamines to trigger theft-ferroptosis in bronchial epithelium.J Clin Invest. 2018 Oct 1;128(10):4639-4653. doi: 10.1172/JCI99490. Epub 2018 Sep 10. J Clin Invest. 2018. PMID: 30198910 Free PMC article.
-
Deuterated Arachidonic Acid Ameliorates Lipopolysaccharide-Induced Lung Damage in Mice.Antioxidants (Basel). 2022 Mar 31;11(4):681. doi: 10.3390/antiox11040681. Antioxidants (Basel). 2022. PMID: 35453366 Free PMC article.
-
The Reaction Specificity of Mammalian ALOX15 Orthologs is Changed During Late Primate Evolution and These Alterations Might Offer Evolutionary Advantages for Hominidae.Front Cell Dev Biol. 2022 Apr 21;10:871585. doi: 10.3389/fcell.2022.871585. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35531094 Free PMC article.
References
-
- Andreou A., Brodhun F., Feussner I. Biosynthesis of oxylipins in non-mammals. Prog. Lipid Res. 2009;48:148–170. - PubMed
-
- Haeggstrom J.Z., Funk C.D. Lipoxygenase and leukotriene pathways: biochemistry, biology, and roles in disease. Chem. Rev. 2011;111:5866–5898. - PubMed
-
- de Bentzmann S., Plesiat P. The Pseudomonas aeruginosa opportunistic pathogen and human infections. Environ. Microbiol. 2011;13:1655–1665. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

