Extranuclear signaling by sex steroid receptors and clinical implications in breast cancer
- PMID: 29146555
- PMCID: PMC5878997
- DOI: 10.1016/j.mce.2017.11.010
Extranuclear signaling by sex steroid receptors and clinical implications in breast cancer
Abstract
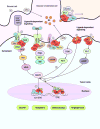
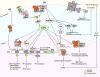
Estrogen and progesterone play essential roles in the development and progression of breast cancer. Over 70% of breast cancers express estrogen receptors (ER) and progesterone receptors (PR), emphasizing the need for better understanding of ER and PR signaling. ER and PR are traditionally viewed as transcription factors that directly bind DNA to regulate gene networks. In addition to nuclear signaling, ER and PR mediate hormone-induced, rapid extranuclear signaling at the cell membrane or in the cytoplasm which triggers downstream signaling to regulate rapid or extended cellular responses. Specialized membrane and cytoplasmic proteins may also initiate hormone-induced extranuclear signaling. Rapid extranuclear signaling converges with its nuclear counterpart to amplify ER/PR transcription and specify gene regulatory networks. This review summarizes current understanding and updates on ER and PR extranuclear signaling. Further investigation of ER/PR extranuclear signaling may lead to development of novel targeted therapeutics for breast cancer management.
Keywords: Breast cancer; Estrogen receptor; Growth factor signaling; Nongenomic signaling; Progesterone receptor; Rapid membrane signaling.
Copyright © 2017 Elsevier B.V. All rights reserved.
Figures
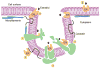
Similar articles
-
Extranuclear expression of hormone receptors in primary breast cancer.Ann Oncol. 2006 Aug;17(8):1213-20. doi: 10.1093/annonc/mdl118. Epub 2006 Jun 7. Ann Oncol. 2006. PMID: 16760268
-
Estrogen and progesterone signalling in the normal breast and its implications for cancer development.Mol Cell Endocrinol. 2018 May 5;466:2-14. doi: 10.1016/j.mce.2017.08.011. Epub 2017 Aug 26. Mol Cell Endocrinol. 2018. PMID: 28851667 Review.
-
Progesterone metabolites regulate induction, growth, and suppression of estrogen- and progesterone receptor-negative human breast cell tumors.Breast Cancer Res. 2013 May 11;15(3):R38. doi: 10.1186/bcr3422. Breast Cancer Res. 2013. PMID: 25927181 Free PMC article.
-
Mediator of ERBB2-driven cell motility (MEMO) promotes extranuclear estrogen receptor signaling involving the growth factor receptors IGF1R and ERBB2.J Biol Chem. 2013 Aug 23;288(34):24590-9. doi: 10.1074/jbc.M113.467837. Epub 2013 Jul 16. J Biol Chem. 2013. PMID: 23861392 Free PMC article.
-
Receptor mechanisms of rapid extranuclear signalling initiated by steroid hormones.Essays Biochem. 2004;40:105-20. doi: 10.1042/bse0400105. Essays Biochem. 2004. PMID: 15242342 Review.
Cited by
-
Steroid Hormone Receptors: Links With Cell Cycle Machinery and Breast Cancer Progression.Front Oncol. 2021 Mar 12;11:620214. doi: 10.3389/fonc.2021.620214. eCollection 2021. Front Oncol. 2021. PMID: 33777765 Free PMC article. Review.
-
Non-Genomic Actions of Estrogens on the DNA Repair Pathways Are Associated With Chemotherapy Resistance in Breast Cancer.Front Oncol. 2021 Mar 19;11:631007. doi: 10.3389/fonc.2021.631007. eCollection 2021. Front Oncol. 2021. PMID: 33869016 Free PMC article. Review.
-
Whole-Genome Omics Elucidates the Role of CCM1 and Progesterone in Cerebral Cavernous Malformations within CmPn Networks.Diagnostics (Basel). 2024 Aug 28;14(17):1895. doi: 10.3390/diagnostics14171895. Diagnostics (Basel). 2024. PMID: 39272679 Free PMC article.
-
Antiestrogens in combination with immune checkpoint inhibitors in breast cancer immunotherapy.J Steroid Biochem Mol Biol. 2019 Oct;193:105415. doi: 10.1016/j.jsbmb.2019.105415. Epub 2019 Jun 19. J Steroid Biochem Mol Biol. 2019. PMID: 31226312 Free PMC article.
-
Sex steroid hormone function in the brain niche: Implications for brain metastatic colonization and progression.Cancer Rep (Hoboken). 2022 Apr;5(4):e1241. doi: 10.1002/cnr2.1241. Epub 2020 Mar 3. Cancer Rep (Hoboken). 2022. PMID: 33350105 Free PMC article. Review.
References
-
- King WJ, Greene GL. Monoclonal antibodies localize oestrogen receptor in the nuclei of target cells. Nature. 1984;307:745–747. - PubMed
-
- Green S, Chambon P. Nuclear receptors enhance our understanding of transcription regulation. Trends Genet. 1988;4:309–314. - PubMed
-
- Jensen EV, Greene GL, Closs LE, DeSombre ER, Nadji M. Receptors reconsidered: a 20-year perspective. Recent Prog Horm Res. 1982;38:1–40. - PubMed
-
- Jensen EV, Jordan VC. The estrogen receptor: a model for molecular medicine. Clinical cancer research: an official journal of the American Association for Cancer Research. 2003;9:1980–1989. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

