Structure-guided engineering of the substrate specificity of a fungal β-glucuronidase toward triterpenoid saponins
- PMID: 29146597
- PMCID: PMC5767852
- DOI: 10.1074/jbc.M117.801910
Structure-guided engineering of the substrate specificity of a fungal β-glucuronidase toward triterpenoid saponins
Abstract
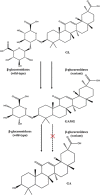
Glycoside hydrolases (GHs) have attracted special attention in research aimed at modifying natural products by partial removal of sugar moieties to manipulate their solubility and efficacy. However, these modifications are challenging to control because the low substrate specificity of most GHs often generates undesired by-products. We previously identified a GH2-type fungal β-glucuronidase from Aspergillus oryzae (PGUS) exhibiting promiscuous substrate specificity in hydrolysis of triterpenoid saponins. Here, we present the PGUS structure, representing the first structure of a fungal β-glucuronidase, and that of an inactive PGUS mutant in complex with the native substrate glycyrrhetic acid 3-O-mono-β-glucuronide (GAMG). PGUS displayed a homotetramer structure with each monomer comprising three distinct domains: a sugar-binding, an immunoglobulin-like β-sandwich, and a TIM barrel domain. Two catalytic residues, Glu414 and Glu505, acted as acid/base and nucleophile, respectively. Structural and mutational analyses indicated that the GAMG glycan moiety is recognized by polar interactions with nine residues (Asp162, His332, Asp414, Tyr469, Tyr473, Asp505, Arg563, Asn567, and Lys569) and that the aglycone moiety is recognized by aromatic stacking and by a π interaction with the four aromatic residues Tyr469, Phe470, Trp472, and Tyr473 Finally, structure-guided mutagenesis to precisely manipulate PGUS substrate specificity in the biotransformation of glycyrrhizin into GAMG revealed that two amino acids, Ala365 and Arg563, are critical for substrate specificity. Moreover, we obtained several mutants with dramatically improved GAMG yield (>95%). Structural analysis suggested that modulating the interaction of β-glucuronidase simultaneously toward glycan and aglycone moieties is critical for tuning its substrate specificity toward triterpenoid saponins.
Keywords: crystal structure; enzyme mutation; fungal β-glucuronidase; glycoside hydrolase; protein engineering; substrate specificity; triterpenoid saponins.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures





References
-
- Verdoucq L., Morinière J., Bevan D. R., Esen A., Vasella A., Henrissat B., and Czjze M. (2004) Structural determinants of substrate specificity in family 1 β-glucosidases. Novel insights from the crystal structure of sorghum dhurrinase-1, a plant β-glucosidase with strict specificity, in complex with its natural substrate. J. Biol. Chem. 279, 31796–31803 10.1074/jbc.M402918200 - DOI - PubMed
-
- Brito-Arias M. (2007) Synthesis and Characterization of Glycosides, pp. 1–67, Springer, Boston, MA
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

