First-in-Human Human Epidermal Growth Factor Receptor 2-Targeted Imaging Using 89Zr-Pertuzumab PET/CT: Dosimetry and Clinical Application in Patients with Breast Cancer
- PMID: 29146695
- PMCID: PMC6004559
- DOI: 10.2967/jnumed.117.202010
First-in-Human Human Epidermal Growth Factor Receptor 2-Targeted Imaging Using 89Zr-Pertuzumab PET/CT: Dosimetry and Clinical Application in Patients with Breast Cancer
Abstract
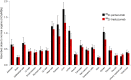
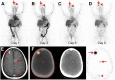
In what we believe to be a first-in-human study, we evaluated the safety and dosimetry of 89Zr-pertuzumab PET/CT for human epidermal growth factor receptor 2 (HER2)-targeted imaging in patients with HER2-positive breast cancer. Methods: Patients with HER2-positive breast cancer and evidence of distant metastases were enrolled in an institutional review board-approved prospective clinical trial. Pertuzumab was conjugated with deferoxamine and radiolabeled with 89Zr. Patients underwent PET/CT with 74 MBq of 89Zr-pertuzumab in a total antibody mass of 20-50 mg of pertuzumab. PET/CT, whole-body probe counts, and blood drawing were performed over 8 d to assess pharmacokinetics, biodistribution, and dosimetry. PET/CT images were evaluated for the ability to visualize HER2-positive metastases. Results: Six patients with HER2-positive metastatic breast cancer were enrolled and administered 89Zr-pertuzumab. No toxicities occurred. Dosimetry estimates from OLINDA demonstrated that the organs receiving the highest doses (mean ± SD) were the liver (1.75 ± 0.21 mGy/MBq), the kidneys (1.27 ± 0.28 mGy/MBq), and the heart wall (1.22 ± 0.16 mGy/MBq), with an average effective dose of 0.54 ± 0.07 mSv/MBq. PET/CT demonstrated optimal imaging 5-8 d after administration. 89Zr-pertuzumab was able to image multiple sites of malignancy and suggested that they were HER2-positive. In 2 patients with both known HER2-positive and HER2-negative primary breast cancers and brain metastases, 89Zr-pertuzumab PET/CT suggested that the brain metastases were HER2-positive. In 1 of the 2 patients, subsequent resection of a brain metastasis proved HER2-positive disease, confirming that the 89Zr-pertuzumab avidity was a true-positive result for HER2-positive malignancy. Conclusion: This first-in-human study demonstrated safety, dosimetry, biodistribution, and successful HER2-targeted imaging with 89Zr-pertuzumab PET/CT. Potential clinical applications include assessment of the HER2 status of lesions that may not be accessible to biopsy and assessment of HER2 heterogeneity.
Keywords: 89Zr-pertuzumab; PET/CT; breast cancer; dosimetry; oncology; radiobiology.
© 2018 by the Society of Nuclear Medicine and Molecular Imaging.
Figures


References
-
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. - PubMed
-
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. - PubMed
-
- Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
