Humanized mice in studying efficacy and mechanisms of PD-1-targeted cancer immunotherapy
- PMID: 29146734
- PMCID: PMC5892726
- DOI: 10.1096/fj.201700740R
Humanized mice in studying efficacy and mechanisms of PD-1-targeted cancer immunotherapy
Abstract
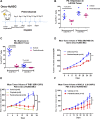
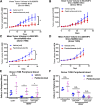
Establishment of an in vivo small animal model of human tumor and human immune system interaction would enable preclinical investigations into the mechanisms underlying cancer immunotherapy. To this end, nonobese diabetic (NOD).Cg- PrkdcscidIL2rgtm1Wjl/Sz (null; NSG) mice were transplanted with human (h)CD34+ hematopoietic progenitor and stem cells, which leads to the development of human hematopoietic and immune systems [humanized NSG (HuNSG)]. HuNSG mice received human leukocyte antigen partially matched tumor implants from patient-derived xenografts [PDX; non-small cell lung cancer (NSCLC), sarcoma, bladder cancer, and triple-negative breast cancer (TNBC)] or from a TNBC cell line-derived xenograft (CDX). Tumor growth curves were similar in HuNSG compared with nonhuman immune-engrafted NSG mice. Treatment with pembrolizumab, which targets programmed cell death protein 1, produced significant growth inhibition in both CDX and PDX tumors in HuNSG but not in NSG mice. Finally, inhibition of tumor growth was dependent on hCD8+ T cells, as demonstrated by antibody-mediated depletion. Thus, tumor-bearing HuNSG mice may represent an important, new model for preclinical immunotherapy research.-Wang, M., Yao, L.-C., Cheng, M., Cai, D., Martinek, J., Pan, C.-X., Shi, W., Ma, A.-H., De Vere White, R. W., Airhart, S., Liu, E. T., Banchereau, J., Brehm, M. A., Greiner, D. L., Shultz, L. D., Palucka, K., Keck, J. G. Humanized mice in studying efficacy and mechanisms of PD-1-targeted cancer immunotherapy.
Keywords: checkpoint inhibitor; mouse model; patient-derived xenograft; pembrolizumab.
Conflict of interest statement
The authors thank the Flow Contract Site Laboratory (Bothell, WA, USA) for processing and staining samples for flow cytometry, and Pure Transplant Solutions (Oklahoma City, OK, USA) for HLA typing. This study was funded by The Jackson Laboratory. The authors declare no conflicts of interest.
Figures




References
-
- Hackam D. G., Redelmeier D. A. (2006) Translation of research evidence from animals to humans. JAMA 296, 1731–1732 https://doi.org/10.1001/jama.296.14.1731 - DOI - PubMed
-
- Mestas J., Hughes C. C. (2004) Of mice and not men: differences between mouse and human immunology. J. Immunol. 172, 2731–2738 https://doi.org/10.4049/jimmunol.172.5.2731 - DOI - PubMed
-
- Theocharides A. P., Rongvaux A., Fritsch K., Flavell R. A., Manz M. G. (2016) Humanized hemato-lymphoid system mice. Haematologica 101, 5–19 https://doi.org/10.3324/haematol.2014.115212 - DOI - PMC - PubMed
-
- Rongvaux A., Willinger T., Martinek J., Strowig T., Gearty S. V., Teichmann L. L., Saito Y., Marches F., Halene S., Palucka A. K., Manz M. G., Flavell R. A. (2014) Development and function of human innate immune cells in a humanized mouse model. Nat. Biotechnol. 32, 364–372 https://doi.org/10.1038/nbt.2858 - DOI - PMC - PubMed
-
- Ishikawa F., Yasukawa M., Lyons B., Yoshida S., Miyamoto T., Yoshimoto G., Watanabe T., Akashi K., Shultz L. D., Harada M. (2005) Development of functional human blood and immune systems in NOD/SCID/IL2 receptor gamma chain(null) mice. Blood 106, 1565–1573 https://doi.org/10.1182/blood-2005-02-0516 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

