Glyoxal as an alternative fixative to formaldehyde in immunostaining and super-resolution microscopy
- PMID: 29146773
- PMCID: PMC5753035
- DOI: 10.15252/embj.201695709
Glyoxal as an alternative fixative to formaldehyde in immunostaining and super-resolution microscopy
Abstract
Paraformaldehyde (PFA) is the most commonly used fixative for immunostaining of cells, but has been associated with various problems, ranging from loss of antigenicity to changes in morphology during fixation. We show here that the small dialdehyde glyoxal can successfully replace PFA Despite being less toxic than PFA, and, as most aldehydes, likely usable as a fixative, glyoxal has not yet been systematically tried in modern fluorescence microscopy. Here, we tested and optimized glyoxal fixation and surprisingly found it to be more efficient than PFA-based protocols. Glyoxal acted faster than PFA, cross-linked proteins more effectively, and improved the preservation of cellular morphology. We validated glyoxal fixation in multiple laboratories against different PFA-based protocols and confirmed that it enabled better immunostainings for a majority of the targets. Our data therefore support that glyoxal can be a valuable alternative to PFA for immunostaining.
Keywords: PFA; fixation; glyoxal; immunocytochemistry; super‐resolution Microscopy.
© 2017 The Authors. Published under the terms of the CC BY 4.0 license.
Figures
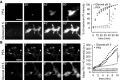
Speed of propidium iodide (PI) penetration into fibroblasts during 60 min of fixation with either 4% PFA or 3% glyoxal. N = 3 independent experiments. Glyoxal fixation enables PI to penetrate far more rapidly into the cells.
Speed of FM 1‐43 penetration in similar experiments. The arrowhead points to one example of ongoing endocytosis during PFA fixation. N = 3–4 independent experiments. The general pattern of FM 1‐43 entry was similar to that of propidium iodide. Only the first 10 min are shown, to enable an optimal observation of the kinetics of the first stages of FM 1‐43 entry. The results parallel those obtained with PI: faster penetration during glyoxal fixation.
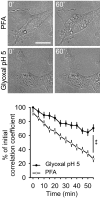
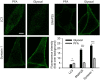
SDS–PAGE gel showing rat brain cytoplasm incubated for 60 min with different fixatives. The graph shows the summed intensity of the bands in each lane. Fixed proteins either no longer run into the gel or form only smears. To compare the efficiency of fixation, the bands that survive fixation were summed and were expressed as % of an unfixed control. The intensity of PFA‐fixed samples was significantly higher than that of glyoxal‐fixed samples (N = 5 independent experiments; one‐way ANOVA with post hoc Tukey test). Glut = 0.2% glutaraldehyde.
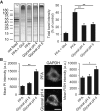
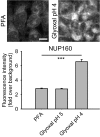
Staining of nucleic acids after fixation. The propidium iodide signal in fibroblasts was significantly higher for samples fixed with glyoxal pH 4 (N = 6–8). To test whether the fixed nucleic acids were still available for specific detection, we performed FISH for GAPDH in cultured neurons, using a standard protocol provided by the company Affymetrix. The fluorescence signal of the samples fixed with glyoxal (pH 4) was significantly higher than for PFA‐fixed samples (N = 5–6; two‐sided Student's t‐test).
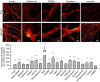
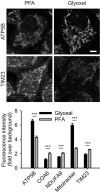
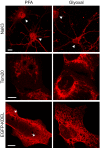
Primary hippocampal neurons were stained for actin and ankyrin G. A comparison between PFA‐ and glyoxal‐fixed samples shows that actin staining with phalloidin works as least as well in both, showing the prominent actin rings. Ankyrin G staining is brighter in PFA‐fixed cells.
Primary hippocampal neurons were stained for pan‐Nav and Kv7.2. Both stainings seem to work at least as well for glyoxal‐fixed neurons as for PFA‐fixed neurons. Staining of K‐channels shows a slightly more regular pattern in glyoxal‐fixed neurons.
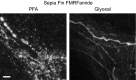
Primary hippocampal neurons were stained for neurofilament L and beta II spectrin. While the spectrin staining seems to be equally well in both fixation conditions, neurofilament staining is brighter in glyoxal‐fixed cells.
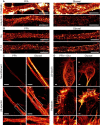
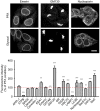
Growth cones of hippocampal neurons were stained for actin and βIII‐tubulin after either glyoxal or PFA + glutaraldehyde fixation. The latter is a standard fixation used for the co‐labeling of tubulin and actin and is a stronger fixation than normal PFA fixation, which is incompatible with many organelle immunostainings (unlike glyoxal fixation). The filopodia and lamellipodia of the growth cones seem to be well stained for the samples fixed with glyoxal, whereas the samples fixed with PFA and glutaraldehyde seem to have lost some of the finer actin structures. Tubulin seems to be a bit better stained in samples fixed with PFA and glutaraldehyde.

- A, B
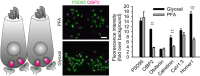
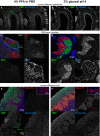
Confocal images showing staining of olfactory marker protein (OMP) and β3‐tubulin along the dorsal aspect of the mouse olfactory epithelium. While sections from both types of fixative show OMP signal in the olfactory sensory neuron somata, their dendrites, and axons, the axon bundles (green arrow) located above the olfactory epithelium exemplify the clear signal‐to‐noise ratio benefits of glyoxal fixation versus that of the PFA‐fixative. Immunostaining with the β3‐tubulin antibody stains the dendrites and axons (blue arrowheads) in both PFA and glyoxal‐fixed tissue, but strong staining of the cilia (blue arrows) can only be observed in the glyoxal‐fixed sections (B).
- C, D
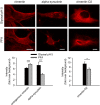
Confocal images depicting bundles of axons belonging to olfactory sensory neurons on the path toward the olfactory bulb. Identities of the axons are in part defined by the neuropilin‐1 (Nrp1) and neuropilin‐2 (Nrp2) expression levels, visualized here with antibodies raised against the two proteins. While complementary expression of the two molecules can be seen in the PFA‐fixed sections (C), the glyoxal‐fixed sections (D) exhibit profoundly improved signal‐to‐noise ratios for Nrp‐1 (red arrows), and in the case of Nrp‐2, also the segmentation of the axon bundle into varying levels of Nrp‐2 (green arrows).
- E, F
Confocal images of olfactory sensory neuron axons coalescing into glomeruli where they synapse with dendrites of olfactory bulb neurons. The axons of olfactory sensory neurons can be readily visualized with OMP staining (green) in the superficial olfactory nerve layer and terminating in glomeruli located below (green arrows). While sections fixed with either PFA or glyoxal display adequate staining levels, the signal distribution of the PFA‐fixed tissue appears more irregular (E), seemingly lacking the neurofilamentary morphology that appears preserved in the glyoxal‐fixed sections (F). The glomeruli themselves are neuropil structures comprised primarily of olfactory sensory neurons forming synapses with dendrites of mitral/tufted cells as well as dendrites of periglomerular neurons. Immunostaining with vesicular glutamate transporter 2 (VGLUT2) allows visualization of these structures and is easily seen in the glyoxal‐fixed section (F), while it appears the antigen was masked by PFA fixation as no signal above background can be seen in the PFA‐fixed panel (red arrows in E). Note that a different polyclonal antibody for VGLUT2 from the same provider does provide signal with PFA, albeit weaker versus glyoxal (inset). Staining with β3‐tubulin touts the benefits of glyoxal both due to the signal improvements in the case of the mild staining in the axons of the olfactory nerve layer that is only visible in the glyoxal‐fixed tissue, but also in preserving tissue morphology as demonstrated by the dendritic processes inside glomeruli (blue arrows) and in the external plexiform layer located below the glomeruli.

References
-
- Beaudoin GMJ, Lee S‐H, Singh D, Yuan Y, Ng Y‐G, Reichardt LF, Arikkath J (2012) Culturing pyramidal neurons from the early postnatal mouse hippocampus and cortex. Nat Protoc 7: 1741–1754 - PubMed
-
- Boucher J, Simard É, Froehlich U, D'Orléans‐Juste P, Grandbois M (2015) Using carboxyfluorescein diacetate succinimidyl ester to monitor intracellular protein glycation. Anal Biochem 478: 73–81 - PubMed
-
- Brunelle JL, Green R (2014) One‐dimensional SDS‐polyacrylamide gel electrophoresis (1D SDS‐PAGE), 1st edn Amsterdam, the Netherlands: Elsevier Inc. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

