α-Perfluoroalkyl-β-alkynylation of alkenes via radical alkynyl migration
- PMID: 29147514
- PMCID: PMC5637125
- DOI: 10.1039/c7sc02175e
α-Perfluoroalkyl-β-alkynylation of alkenes via radical alkynyl migration
Abstract
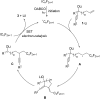
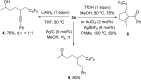
Transition metal-free radical α-perfluoroalkylation with concomitant β-alkynylation of unactivated alkenes is presented. These cascades proceed via electron-catalysis and comprise a radical 1,4- or 1,5-alkynyl migration from tertiary propargylic alcoholates to secondary or tertiary C-radicals as the key step. Alkynyl migration leads to a ketyl radical anion that sustains the chain as a single electron transfer reducing reagent.
Figures


References
-
- Kirk K. L. Org. Process Res. Dev. 2008;12:305–321.
-
- Müller K., Faeh C., Diederich F. Science. 2007;317:1881–1886. - PubMed
-
- Li Y., Studer A. Angew. Chem., Int. Ed. 2012;51:8221–8224. - PubMed
- Hartmann M., Li Y., Studer A. J. Am. Chem. Soc. 2012;134:16516–16519. - PubMed
- Zhang L., Li Z., Liu Z.-Q. Org. Lett. 2014;16:3688–3691. - PubMed
- Yang N.-Y., Li Z.-L., Tan B., Liu X.-Y. Chem. Commun. 2016;52:9052–9055. - PubMed
- Wu Z., Wang D., Liu Y., Hua L., Zhu C. J. Am. Chem. Soc. 2017;139:1388–1391. - PubMed
-
- Mu X., Wu T., Wang H.-Y., Guo Y.-L., Liu G. J. Am. Chem. Soc. 2012;134:878–881. - PubMed
- Egami H., Shimizu R., Sodeoka M. J. Fluorine Chem. 2013;152:51–55.
- Dong X., Sang R., Wang Q., Tang X.-Y., Shi M. Chem.–Eur. J. 2013;19:16910–16915. - PubMed
- Liu X., Xiong F., Huang X., Xu L., Li P., Wu X. Angew. Chem., Int. Ed. 2013;52:6962–6966. - PubMed
- Egami H., Shimizu R., Kawamura S., Sodeoka M. Angew. Chem., Int. Ed. 2013;52:4000–4003. - PubMed
- Kong W., Casimiro M., Merino E., Nevado C. J. Am. Chem. Soc. 2013;135:14480–14483. - PubMed
- Chen Z.-M., Bai W., Wang S.-H., Yang B.-M., Tu Y.-Q., Zhang F.-M. Angew. Chem., Int. Ed. 2013;52:9781–9785. - PubMed
- Han G., Wang Q., Liu Y., Wang Q. Org. Lett. 2014;16:5914–5917. - PubMed
- Wang F., Wang D., Mu X., Chen P., Liu G. J. Am. Chem. Soc. 2014;136:10202–10205. - PubMed
- He Y.-T., Li L.-H., Zhou Z.-Z., Hua H.-L., Qiu Y.-F., Liu X.-Y., Liang Y.-M. Org. Lett. 2014;16:3896–3899. - PubMed
- Yang B., Xu X.-H., Qing F.-L. Org. Lett. 2015;17:1906–1909. - PubMed
- Zheng J., Deng Z., Zhang Y., Cui S. Adv. Synth. Catal. 2016;358:746–751.
- Guo J.-Y., Wu R.-X., Jin J.-K., Tian S.-K. Org. Lett. 2016;18:3850–3853. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

