Development of proteolytically stable N-methylated peptide inhibitors of aggregation of the amylin peptide implicated in type 2 diabetes
- PMID: 29147551
- PMCID: PMC5665791
- DOI: 10.1098/rsfs.2016.0127
Development of proteolytically stable N-methylated peptide inhibitors of aggregation of the amylin peptide implicated in type 2 diabetes
Abstract
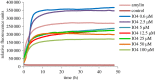
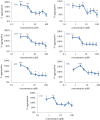
Islet amyloid polypeptide, also known as amylin, is the main component of the amyloid deposits present in approximately 90% of people with type 2 diabetes mellitus (T2DM). In this disease, amylin aggregates into multimeric β-pleated sheet structures which cause damage to pancreatic islet β-cells. Inhibitors of early-stage amylin aggregation could therefore provide a disease-modifying treatment for T2DM. In this study, overlapping peptides were designed to target the 'binding' region (RLANFLVHSS, residues 11-20) of human amylin, and their effects on amyloid fibril formation were determined by thioflavin-T assay. The first generation peptides showed less than 50% inhibition of aggregation, but a second generation peptide (H2N-RGANFLVHGR-CONH2) showed strong inhibitory effects on amylin aggregation, and this was confirmed by negative stain electron microscopy. Cytotoxicity studies revealed that this peptide protected human pancreatic 1.4E7 (ECACC 10070102) insulin-secreting cells from the toxic effects of human amylin. Unlike the retro-inverso version of this peptide, which stimulated aggregation, two N-methylated peptides (H2N-RGAmNFmLVmHGR-CONH2 and H2N-RGANmFLmVHmR-CONH2) gave very clear dose-dependent inhibition of fibril formation. These two peptides were also stable against a range of different proteolytic enzymes, and in human plasma. These N-methylated peptides could provide a novel treatment for slowing progression of T2DM.
Keywords: IAPP; N-methylated peptide; amylin; amyloid; diabetes; islet amyloid polypeptide.
Conflict of interest statement
We declare we have no competing interests.
Figures





References
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
