Cell death and cell lysis are separable events during pyroptosis
- PMID: 29147575
- PMCID: PMC5682879
- DOI: 10.1038/cddiscovery.2017.70
Cell death and cell lysis are separable events during pyroptosis
Abstract
Although much insight has been gained into the mechanisms by which activation of the inflammasome can trigger pyroptosis in mammalian cells, the precise kinetics of the end stages of pyroptosis have not been well characterized. Using time-lapse fluorescent imaging to analyze the kinetics of pyroptosis in individual murine macrophages, we observed distinct stages of cell death and cell lysis. Our data demonstrate that cell membrane permeability resulting from gasdermin D pore formation is coincident with the cessation of cell movement, loss of mitochondrial activity, and cell swelling, events that can be uncoupled from cell lysis. We propose a model of pyroptosis in which cell death can occur independently of cell lysis. The uncoupling of cell death from cell lysis may allow for better control of cytosolic contents upon activation of the inflammasome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
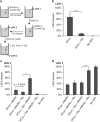
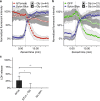
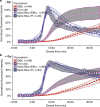



References
-
- Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015; 526: 660–665. - PubMed
-
- Kayagaki N, Stowe IB, Lee BL, O'Rourke K, Anderson K, Warming S et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 2015; 526: 666–671. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

