Regulation of Signal Transduction by DJ-1
- PMID: 29147906
- PMCID: PMC5821264
- DOI: 10.1007/978-981-10-6583-5_8
Regulation of Signal Transduction by DJ-1
Abstract
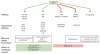
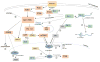
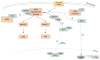
The ability of DJ-1 to modulate signal transduction has significant effects on how the cell regulates normal processes such as growth, senescence, apoptosis, and autophagy to adapt to changing environmental stimuli and stresses. Perturbations of DJ-1 levels or function can disrupt the equilibrium of homeostatic signaling networks and set off cascades that play a role in the pathogenesis of conditions such as cancer and Parkinson's disease.DJ-1 plays a major role in various pathways. It mediates cell survival and proliferation by activating the extracellular signal-regulated kinase (ERK1/2) pathway and the phosphatidylinositol-3-kinase (PI3K)/Akt pathway. It attenuates cell death signaling by inhibiting apoptosis signal-regulating kinase 1 (ASK1) activation as well as by inhibiting mitogen-activated protein kinase kinase kinase 1 (MEKK1/MAP3K1) activation of downstream apoptotic cascades. It also modulates autophagy through the ERK, Akt, or the JNK/Beclin1 pathways. In addition, DJ-1 regulates the transcription of genes essential for male reproductive function, such as spermatogenesis, by relaying nuclear receptor androgen receptor (AR) signaling. In this chapter, we summarize the ways that DJ-1 regulates these pathways, focusing on how its role in signal transduction contributes to cellular homeostasis and the pathologic states that result from dysregulation.
Keywords: AR; ASK1; Akt; Cell signaling; DJ-1; Daxx; ERK; JNK; MAPK; MEK; MEKK1; PI3K; Raf; Ras; Signal transduction; Trx1; mTOR; p38.
Figures

Similar articles
-
Cytoprotective mechanisms of DJ-1 against oxidative stress through modulating ERK1/2 and ASK1 signal transduction.Redox Biol. 2018 Apr;14:211-217. doi: 10.1016/j.redox.2017.09.008. Epub 2017 Sep 18. Redox Biol. 2018. PMID: 28954246 Free PMC article. Review.
-
DJ-1 modulates the p38 mitogen-activated protein kinase pathway through physical interaction with apoptosis signal-regulating kinase 1.J Cell Biochem. 2010 May;110(1):229-37. doi: 10.1002/jcb.22530. J Cell Biochem. 2010. PMID: 20213747
-
Interaction of DJ-1 with Daxx inhibits apoptosis signal-regulating kinase 1 activity and cell death.Proc Natl Acad Sci U S A. 2005 Jul 5;102(27):9691-6. doi: 10.1073/pnas.0409635102. Epub 2005 Jun 27. Proc Natl Acad Sci U S A. 2005. PMID: 15983381 Free PMC article.
-
The Parkinson's disease gene product DJ-1 modulates miR-221 to promote neuronal survival against oxidative stress.Redox Biol. 2018 Oct;19:62-73. doi: 10.1016/j.redox.2018.07.021. Epub 2018 Aug 3. Redox Biol. 2018. PMID: 30107296 Free PMC article.
-
Modulation of signaling pathways by DJ-1: An updated overview.Redox Biol. 2022 May;51:102283. doi: 10.1016/j.redox.2022.102283. Epub 2022 Mar 11. Redox Biol. 2022. PMID: 35303520 Free PMC article. Review.
Cited by
-
Persulfidation of DJ-1: Mechanism and Consequences.Biomolecules. 2022 Dec 22;13(1):27. doi: 10.3390/biom13010027. Biomolecules. 2022. PMID: 36671412 Free PMC article.
-
Effects of DJ‑1 on apoptosis and mitophagy of glomerular podocytes.Exp Ther Med. 2023 Aug 11;26(4):463. doi: 10.3892/etm.2023.12162. eCollection 2023 Oct. Exp Ther Med. 2023. PMID: 37664676 Free PMC article.
-
Chlamydia Infection Remodels Host Cell Mitochondria to Alter Energy Metabolism and Subvert Apoptosis.Microorganisms. 2023 May 24;11(6):1382. doi: 10.3390/microorganisms11061382. Microorganisms. 2023. PMID: 37374883 Free PMC article. Review.
-
The interplay between oxidative stress and autophagy: focus on the development of neurological diseases.Behav Brain Funct. 2022 Jan 29;18(1):3. doi: 10.1186/s12993-022-00187-3. Behav Brain Funct. 2022. PMID: 35093121 Free PMC article. Review.
-
Gami-Chunggan Formula Prevents Motor Dysfunction in MPTP/p-Induced and A53T α-Synuclein Overexpressed Parkinson's Disease Mouse Model Though DJ-1 and BDNF Expression.Front Aging Neurosci. 2019 Aug 28;11:230. doi: 10.3389/fnagi.2019.00230. eCollection 2019. Front Aging Neurosci. 2019. PMID: 31555122 Free PMC article.
References
-
- Abou-Sleiman PM, et al. The role of pathogenic DJ-1 mutations in Parkinson’s disease. Ann Neurol. 2003;54(3):283–286. - PubMed
-
- Abraham D, et al. Raf-1-associated protein phosphatase 2A as a positive regulator of kinase activation. J Biol Chem. 2000;275(29):22300–22304. - PubMed
-
- Adams DG, et al. Positive regulation of Raf1-MEK1/2-ERK1/2 signaling by protein serine/ threonine phosphatase 2A holoenzymes. J Biol Chem. 2005;280(52):42644–42654. - PubMed
-
- Alessi DR, et al. Inactivation of p42 MAP kinase by protein phosphatase 2A and a protein tyrosine phosphatase, but not CL100, in various cell lines. Current Biology. 1995;5(3):283–295. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

