Artificial bio-nanomachines based on protein needles derived from bacteriophage T4
- PMID: 29147941
- PMCID: PMC5899697
- DOI: 10.1007/s12551-017-0336-9
Artificial bio-nanomachines based on protein needles derived from bacteriophage T4
Abstract
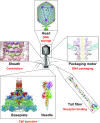
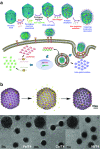
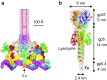
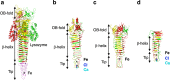
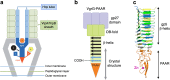
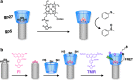
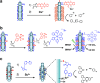
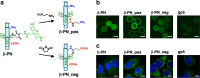
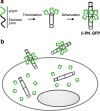
Bacteriophage T4 is a natural bio-nanomachine which achieves efficient infection of host cells via cooperative motion of specific three-dimensional protein architectures. The relationships between the protein structures and their dynamic functions have recently been clarified. In this review we summarize the design principles for fabrication of nanomachines using the component proteins of bacteriophage T4 based on these recent advances. We focus on the protein needle known as gp5, which is located at the center of the baseplate at the end of the contractile tail of bacteriophage T4. This protein needle plays a critical role in directly puncturing host cells, and analysis has revealed that it contains a common motif used for cell puncture in other known injection systems, such as T6SS. Our artificial needle based on the β-helical domain of gp5 retains the ability to penetrate cells and can be engineered to deliver various cargos into living cells. Thus, the unique components of bacteriophage T4 and other natural nanomachines have great potential for use as molecular scaffolds in efforts to fabricate new bio-nanomachines.
Keywords: Bacteriophage T4; Cell penetration; Gp5; Protein needle; β-Helix.
Conflict of interest statement
Conflict of interest
Hiroshi Inaba declares that he has no conflict of interest. Takafumi Ueno declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by the authors.
Figures







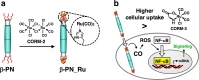
References
-
- Aksyuk AA, Bowman VD, Kaufmann B, Fields C, Klose T, Holdaway HA, Fischetti VA, Rossmann MG. Structural investigations of a Podoviridae streptococcus phage C1, implications for the mechanism of viral entry. Proc Natl Acad Sci USA. 2012;109:14001–14006. doi: 10.1073/pnas.1207730109. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

