Minor salivary gland fibrosis in Sjögren's syndrome is elevated, associated with focus score and not solely a consequence of aging
- PMID: 29148407
- PMCID: PMC5913007
Minor salivary gland fibrosis in Sjögren's syndrome is elevated, associated with focus score and not solely a consequence of aging
Abstract
Objectives: Evaluate the presence of minor salivary gland (SG) fibrosis in primary Sjögren's syndrome (pSS) as a function of disease pathology or a consequence of ageing.
Methods: Subjects with sicca symptoms attending a Sjögren's research clinic were classified by American European Consensus Group (AECG) criteria as either pSS or non-SS (nSS). Discovery (n=34 pSS, n=28 nSS) and replication (n=35 pSS, n=31 nSS) datasets were evaluated. Minor SG cross-sections from haematoxylin and eosin stained slides were imaged, digitally reconstructed and analysed for percent area fibrosis. Relationships between SG fibrosis, age, and clinical measures were evaluated using Spearman correlations. Association with SS was assessed by: ROC curve, Variable Selection Using Random Forests (VSURF) and uni- and bi-variate regression analyses.
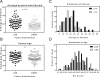
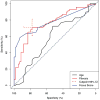
Results: SS subjects had significantly more fibrotic tissue in their minor labial salivary glands (median 24.39%, range 5.12-51.67%) than nSS participants (median 16.7%, range 5.97-38.65%, p<0.0001); age did not differ between groups (average ± SD pSS 50.2 ±13.9 years, nSS 53.8±12.4 years). In both the discovery and replication data sets, multiple regression models showed that the area of minor salivary gland fibrosis predicted pSS significantly better than age alone. Age-corrected linear regression revealed that the area of minor salivary gland fibrosis positively associated with vanBijsterveld score (p=0.042) and biopsy focus score (p=0.002). ROC curve and VSURF analyses ranked fibrosis as a significantly more important variable for subject discrimination than age.
Conclusions: SG fibrosis is an element of pSS pathology that is related to focus score and is not solely attributable to age.
Figures


References
-
- Jonsson R, Vogelsang P, Volchenkov R, Espinosa A, Wahren-Herlenius M, Appel S. The complexity of sjogren's syndrome: Novel aspects on pathogenesis. Immunology letters. 2011;1:1–9. - PubMed
-
- Mavragani CP, Moutsopoulos HM. Sjogren's syndrome. Annu Rev Pathol. 2014:273–85. - PubMed
-
- Nocturne G, Mariette X. Advances in understanding the pathogenesis of primary sjogren's syndrome. Nature reviews. Rheumatology. 2013;9:544–56. - PubMed
-
- Li Y, Zhang K, Chen H, et al. A genome-wide association study in han chinese identifies a susceptibility locus for primary sjogren's syndrome at 7q11.23. Nat Genet. 2013;11:1361–5. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
