An in vitro prototype of a porcine biomimetic testis-like cell culture system: a novel tool for the study of reassembled Sertoli and Leydig cells
- PMID: 29148520
- PMCID: PMC5858101
- DOI: 10.4103/aja.ja_47_17
An in vitro prototype of a porcine biomimetic testis-like cell culture system: a novel tool for the study of reassembled Sertoli and Leydig cells
Abstract
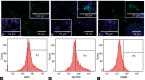
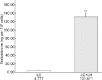
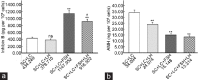
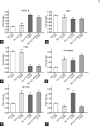
At present, there is no reliable in vitro assembled prepubertal testis-like biomimetic organ culture system designed to assess the functional effects of human gonadotropins on Sertoli and Leydig cells. Spermatogenesis is regulated by endocrine, paracrine, and juxtacrine factors (testicular cross-talk), mainly orchestrated by gonadotropins such as luteinizing hormone (LH) and follicle-stimulating hormone (FSH) that play a pivotal role by stimulating Leydig and Sertoli cells, respectively. The aim of our study was to set up an in vitro prepubertal porcine bioengineered construct as a new model for experimental studies on reassembled Sertoli and Leydig cells. We have evaluated Sertoli and Leydig cells obtained from 15- to 20-day-old neonatal pig testes in terms of purity and function. Subsequently, purified Sertoli and enriched Leydig cells were subjected to coincubation to obtain an in vitro prepubertal porcine testis-like culture system. We performed enzyme-linked immunosorbent assay (ELISA) for anti-Müllerian hormone (AMH), inhibin B, and testosterone secretion in the medium, and Real-Time PCR analysis of AMH, inhibin B, FSH-r, aromatase, LHr, and 3β-HSD mRNA expression levels. This in vitro testis-like system was highly responsive to the effects of human gonadotropins and testosterone. AMH mRNA expression and secretion declined, and inhibin-B increased, while FSH-receptor expression was downregulated upon FSH/LH exposure/treatment. Finally, the production of testosterone was increased selectively upon LH treatment. In summary, our proposed model could help to better determine the action of human gonadotropins on Sertoli and Leydig cells. The potential usefulness of the system for shedding light into male infertility-related issues is evident.
Keywords: Leydig cells; Sertoli cells; human gonadotropins; prepubertal biomimetic testis.
Figures

Similar articles
-
Effect of anti-Mullerian hormone on Sertoli and Leydig cell functions in fetal and immature rats.Endocrinology. 1998 Mar;139(3):1213-20. doi: 10.1210/endo.139.3.5785. Endocrinology. 1998. PMID: 9492056
-
Effects of human recombinant luteinizing hormone and follicle-stimulating hormone in patients with acquired hypogonadotropic hypogonadism: study of Sertoli and Leydig cell secretions and interactions.J Clin Endocrinol Metab. 2000 Sep;85(9):3239-44. doi: 10.1210/jcem.85.9.6811. J Clin Endocrinol Metab. 2000. PMID: 10999815 Clinical Trial.
-
Anti-Muellerian hormone, inhibin A, gonadotropins, and gonadotropin receptors in bull calves after partial scrotal resection, orchidectomy, and Burdizzo castration.Theriogenology. 2017 Jan 1;87:242-249. doi: 10.1016/j.theriogenology.2016.08.030. Epub 2016 Sep 7. Theriogenology. 2017. PMID: 27693012 Clinical Trial.
-
Modulation of steroidogenic activities in testis Leydig cells.Mol Cell Endocrinol. 1981 Jan;21(1):15-28. doi: 10.1016/0303-7207(81)90026-5. Mol Cell Endocrinol. 1981. PMID: 6259000 Review.
-
Paracrine regulation of testicular function.J Steroid Biochem. 1987;27(1-3):317-29. doi: 10.1016/0022-4731(87)90323-2. J Steroid Biochem. 1987. PMID: 3121919 Review.
Cited by
-
Effects of Titanium Dioxide Nanoparticles on Porcine Prepubertal Sertoli Cells: An "In Vitro" Study.Front Endocrinol (Lausanne). 2022 Jan 3;12:751915. doi: 10.3389/fendo.2021.751915. eCollection 2021. Front Endocrinol (Lausanne). 2022. PMID: 35046890 Free PMC article.
-
In "Vitro" Lps-Stimulated Sertoli Cells Pre-Loaded With Microparticles: Intracellular Activation Pathways.Front Endocrinol (Lausanne). 2021 Jan 7;11:611932. doi: 10.3389/fendo.2020.611932. eCollection 2020. Front Endocrinol (Lausanne). 2021. PMID: 33488524 Free PMC article.
-
Effects of GH and IGF1 on Basal and FSH-Modulated Porcine Sertoli Cells In-Vitro.J Clin Med. 2019 Jun 6;8(6):811. doi: 10.3390/jcm8060811. J Clin Med. 2019. PMID: 31174315 Free PMC article.
-
Exploring Sertoli Cells' Innate Bulwark Role Against Infections: In Vitro Performances on Candida tropicalis Biofilms.Cells. 2025 Mar 26;14(7):495. doi: 10.3390/cells14070495. Cells. 2025. PMID: 40214449 Free PMC article.
-
Effect of EPA on Neonatal Pig Sertoli Cells "In Vitro": A Possible Treatment to Help Maintain Fertility in Pre-Pubertal Boys Undergoing Treatment With Gonado-Toxic Therapies.Front Endocrinol (Lausanne). 2021 May 20;12:694796. doi: 10.3389/fendo.2021.694796. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34093450 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

