Molecular testing for BRAF mutations to inform melanoma treatment decisions: a move toward precision medicine
- PMID: 29148538
- PMCID: PMC5758899
- DOI: 10.1038/modpathol.2017.104
Molecular testing for BRAF mutations to inform melanoma treatment decisions: a move toward precision medicine
Abstract
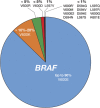
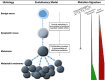
Approximately one-half of advanced (unresectable or metastatic) melanomas harbor a mutation in the BRAF gene, with V600E being the most common mutation. Targeted therapy with BRAF and MEK inhibitors is associated with significant long-term treatment benefit in patients with BRAF V600-mutated melanoma. Therefore, molecular testing for BRAF mutations is a priority in determining the course of therapy. A literature search was performed using MEDLINE/PubMed and scientific congress databases using the terms 'BRAF,' 'mutation,' and 'cancer/tumor.' These results were filtered to include manuscripts that focused on diagnostic tests for determining BRAF mutation status. Numerous BRAF testing methods were identified, including DNA-based companion diagnostic tests and DNA- and protein-based laboratory-developed tests. Herein we review the characteristics of each method and highlight the strengths and weaknesses that should be considered before use and when interpreting results for each patient. Molecular profiling has shown that mutation load increases with melanoma tumor progression and that unique patterns of genetic changes and evolutionary trajectories for different melanoma subtypes can occur. Discordance in the BRAF mutational status between primary and metastatic lesions, as well as intratumoral heterogeneity, is known to occur. Additionally, the development of acquired resistance to combination BRAF and MEK inhibitor therapy is still a formidable obstacle. Therefore, tumor heterogeneity and the development of acquired resistance have important implications for molecular testing and ultimately the treatment of patients with advanced-stage melanoma. Overall, this information may help community oncologists more accurately and effectively interpret results of diagnostic tests within the context of recent data characterizing melanoma tumor progression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- American Cancer Society. Melanoma skin cancer: ACS. Available from http://www.cancer.org/acs/groups/cid/documents/webcontent/003120-pdf.pdf; Accessed 2017.
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. - PubMed
-
- Luke JJ, Flaherty KT, Ribas A et al. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol 2017;14:463–482. - PubMed
-
- Atkins MB, Lotze MT, Dutcher JP et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analylsis of 270 patients treated between 1985 and 1993. J Clin Oncol 1999;17:2105–2116. - PubMed
-
- Schiller JH, Pugh M, Kirkwood JM et al. Eastern Cooperative Group trial of interferon gamma in metastatic melanoma: an innovative study design. Clin Cancer Res 1996;2:29–36. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

