Novel Antitubercular 6-Dialkylaminopyrimidine Carboxamides from Phenotypic Whole-Cell High Throughput Screening of a SoftFocus Library: Structure-Activity Relationship and Target Identification Studies
- PMID: 29148755
- PMCID: PMC5748279
- DOI: 10.1021/acs.jmedchem.7b01347
Novel Antitubercular 6-Dialkylaminopyrimidine Carboxamides from Phenotypic Whole-Cell High Throughput Screening of a SoftFocus Library: Structure-Activity Relationship and Target Identification Studies
Abstract
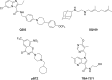
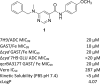
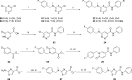
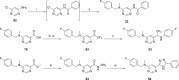
A BioFocus DPI SoftFocus library of ∼35 000 compounds was screened against Mycobacterium tuberculosis (Mtb) in order to identify novel hits with antitubercular activity. The hits were evaluated in biology triage assays to exclude compounds suggested to function via frequently encountered promiscuous mechanisms of action including inhibition of the QcrB subunit of the cytochrome bc1 complex, disruption of cell-wall homeostasis, and DNA damage. Among the hits that passed this screening cascade, a 6-dialkylaminopyrimidine carboxamide series was prioritized for hit to lead optimization. Compounds from this series were active against clinical Mtb strains, while no cross-resistance to conventional antituberculosis drugs was observed. This suggested a novel mechanism of action, which was confirmed by chemoproteomic analysis leading to the identification of BCG_3193 and BCG_3827 as putative targets of the series with unknown function. Initial structure-activity relationship studies have resulted in compounds with moderate to potent antitubercular activity and improved physicochemical properties.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

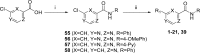
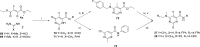



References
-
- World Health Organisation. Global Tuberculosis Report. http://apps.who.int/iris/bitstream/10665/250441/1/9789241565394-eng.pdf?... (accessed Dec 12, 2016).
-
- Andries K.; Verhasselt P.; Guillemont J.; Gohlmann H. W. H.; Neefs J.-M.; Winkler H.; Van Gestel J.; Timmerman P.; Zhu M.; Lee E.; Williams P.; de Chaffoy D.; Huitric E.; Hoffner S.; Cambau E.; Truffot-Pernot C.; Lounis N.; Jarlier V. A Diarylquinoline Drug Active on the ATP Synthase of Mycobacterium Tuberculosis. Science 2005, 307, 223–227. 10.1126/science.1106753. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

