Cyanopivaloyl Ester in the Automated Solid-Phase Synthesis of Oligorhamnans
- PMID: 29148768
- PMCID: PMC5735374
- DOI: 10.1021/acs.joc.7b02511
Cyanopivaloyl Ester in the Automated Solid-Phase Synthesis of Oligorhamnans
Abstract
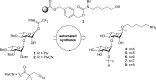
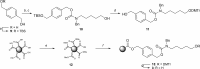
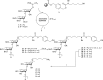
The development of effective protecting group chemistry is an important driving force behind the progress in the synthesis of complex oligosaccharides. Automated solid-phase synthesis is an attractive technique for the rapid assembly of oligosaccharides, built up of repetitive elements. The fact that (harsh) reagents are used in excess in multiple reaction cycles makes this technique extra demanding on the protecting groups used. Here, the synthesis of a set of oligorhamnan fragments is reported using the cyanopivaloyl (PivCN) ester to ensure effective neighboring group participation during the glycosylation events. The PivCN group combines the favorable characteristics of the parent pivaloyl (Piv) ester, stability, minimal migratory aptitude, minimal orthoester formation, while it can be cleaved under mild conditions. We show that the remote CN group in the PivCN renders the PivCN carbonyl more electropositive and thus susceptible to nucleophilic cleavage. This feature is built upon in the automated solid-phase assembly of the oligorhamnan fragments. Where the use of a Piv-protected building block failed because of incomplete cleavage, PivCN-protected synthons performed well and allowed the generation of oligorhamnans, up to 16 monosaccharides in length.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
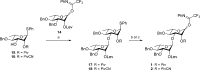
References
-
- Kröck L.; Esposito D.; Castagner B.; Wang C.-C.; Bindschädler P.; Seeberger P. H. Chem. Sci. 2012, 3, 1617.10.1039/c2sc00940d. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

