From bench to almost bedside: the long road to a licensed Ebola virus vaccine
- PMID: 29148858
- PMCID: PMC5841470
- DOI: 10.1080/14712598.2018.1404572
From bench to almost bedside: the long road to a licensed Ebola virus vaccine
Abstract
Introduction: The Ebola virus (EBOV) disease epidemic during 2014-16 in West Africa has accelerated the clinical development of several vaccine candidates that have demonstrated efficacy in the gold standard nonhuman primate (NHP) model, namely cynomolgus macaques.
Areas covered: This review discusses the pre-clinical research and if available, clinical evaluation of the currently available EBOV vaccine candidates, while emphasizing the translatability of pre-clinical data generated in the NHP model to clinical data in humans.
Expert opinion: Despite the existence of many successful EBOV vaccine candidates in the pre-clinical stages, only two platforms became the focus of Phase 2/3 efficacy trials in Liberia, Sierra Leone, and Guinea near the peak of the epidemic: the Vesicular stomatitis virus (VSV)-vectored vaccine and the chimpanzee adenovirus type 3 (ChAd3)-vectored vaccine. The results of three distinct clinical trials involving these candidates may soon pave the way for a licensed, safe and efficacious EBOV vaccine to help combat future epidemics.
Keywords: Clinical trials; ebola virus; nonhuman primates; vaccines.
Conflict of interest statement
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Figures
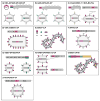
References
-
- Feldmann H, Sanchez A, Geisbert TW. Filoviridae: Marburg and Ebola viruses. In: Knipe DM, Howley P, editors. Fields Virology. Philadelphia, PA, USA: Lippincott Williams and Wilkins; 2013.
-
- Rowe AK, Bertolli J, Khan AS, et al. Clinical, virologic, and immunologic follow-up of convalescent Ebola hemorrhagic fever patients and their household contacts, Kikwit, Democratic Republic of the Congo. Commission de Lutte contre les Epidemies a Kikwit. The Journal of infectious diseases. 1999 Feb;179(Suppl 1):S28–35. doi: 10.1086/514318. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
