Convergence of topological domain boundaries, insulators, and polytene interbands revealed by high-resolution mapping of chromatin contacts in the early Drosophila melanogaster embryo
- PMID: 29148971
- PMCID: PMC5739541
- DOI: 10.7554/eLife.29550
Convergence of topological domain boundaries, insulators, and polytene interbands revealed by high-resolution mapping of chromatin contacts in the early Drosophila melanogaster embryo
Abstract
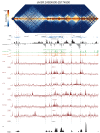
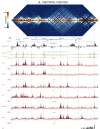
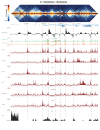
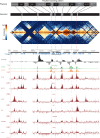
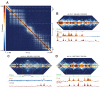
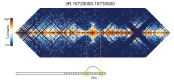
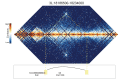
High-throughput assays of three-dimensional interactions of chromosomes have shed considerable light on the structure of animal chromatin. Despite this progress, the precise physical nature of observed structures and the forces that govern their establishment remain poorly understood. Here we present high resolution Hi-C data from early Drosophila embryos. We demonstrate that boundaries between topological domains of various sizes map to DNA elements that resemble classical insulator elements: short genomic regions sensitive to DNase digestion that are strongly bound by known insulator proteins and are frequently located between divergent promoters. Further, we show a striking correspondence between these elements and the locations of mapped polytene interband regions. We believe it is likely this relationship between insulators, topological boundaries, and polytene interbands extends across the genome, and we therefore propose a model in which decompaction of boundary-insulator-interband regions drives the organization of interphase chromosomes by creating stable physical separation between adjacent domains.
Keywords: D. melanogaster; Hi-C; ZGA; chromatin topology; chromosomes; evolutionary biology; gene regulation; genes; genomics; insulator; polytene.
Conflict of interest statement
No competing interests declared.
Figures
























References
-
- Balbiani EG. Sur La Structure Du Noyau Des Cellules Salivares Chez Les Larves de Chironomus. Zoologischer Anzeiger. 1881;4:637662–641666.
-
- Balbiani EG. Sur La Structure Intime Du Noyau Du Loxophyllum Meleagris. Zoologischer Anzeiger. 1890;13:110, 132–15.
-
- Beagrie RA, Scialdone A, Schueler M, Kraemer DC, Chotalia M, Xie SQ, Barbieri M, de Santiago I, Lavitas LM, Branco MR, Fraser J, Dostie J, Game L, Dillon N, Edwards PA, Nicodemi M, Pombo A. Complex multi-enhancer contacts captured by genome architecture mapping. Nature. 2017;543:519–524. doi: 10.1038/nature21411. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

