New Insights into Phasmatodea Chromosomes
- PMID: 29149047
- PMCID: PMC5704240
- DOI: 10.3390/genes8110327
New Insights into Phasmatodea Chromosomes
Abstract
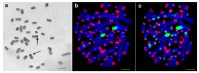
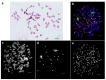
Currently, approximately 3000 species of stick insects are known; however, chromosome numbers, which range between 21 and 88, are known for only a few of these insects. Also, centromere banding staining (C-banding) patterns were described for fewer than 10 species, and fluorescence in situ hybridization (FISH) was applied exclusively in two Leptynia species. Interestingly, 10-25% of stick insects (Phasmatodea) are obligatory or facultative parthenogenetic. As clonal and/or bisexual reproduction can affect chromosomal evolution, stick insect karyotypes need to be studied more intensely. Chromosome preparation from embryos of five Phasmatodea species (Medauroidea extradentata, Sungaya inexpectata, Sipyloidea sipylus, Phaenopharos khaoyaiensis, and Peruphasma schultei) from four families were studied here by C-banding and FISH applying ribosomal deoxyribonucleic acid (rDNA) and telomeric repeat probes. For three species, data on chromosome numbers and structure were obtained here for the first time, i.e., S. inexpectata, P. khaoyaiensis, and P. schultei. Large C-positive regions enriched with rDNA were identified in all five studied, distantly related species. Some of these C-positive blocks were enriched for telomeric repeats, as well. Chromosomal evolution of stick insects is characterized by variations in chromosome numbers as well as transposition and amplification of repetitive DNA sequences. Here, the first steps were made towards identification of individual chromosomes in Phasmatodea.
Keywords: C-banding; Phasmatodea; fluorescence in situ hybridization; interstitial telomeric sequences; ribosomal deoxyribonucleic acid; stick insects; telomeric repeats.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures




References
-
- Conle O., Hennemann F. Studies on neotropical Phasmatodea 1: A remarkable new species of Peruphasma Conle & Hennemann, 2002 from Northern Peru (Phasmatodea: Pseudophasmatidae: Pseudophasmatinae) Zootaxa. 2005;1068:59–68.
-
- Von Wattenwyl K.B. Die Insektenfamilie der Phasmiden, II. Phasmidae Anareolatae. Wilhelm Engelmann; Leipzig, Germany: 1907.
-
- Gregory T.R. Animal Genome Size Database. [(accessed on 20 August 2009)]; Available online: http://www.genomesize.com.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

