BCL-2 family proteins: changing partners in the dance towards death
- PMID: 29149100
- PMCID: PMC5729540
- DOI: 10.1038/cdd.2017.186
BCL-2 family proteins: changing partners in the dance towards death
Abstract
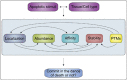
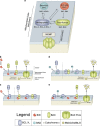
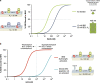
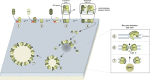
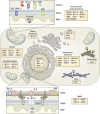
The BCL-2 family of proteins controls cell death primarily by direct binding interactions that regulate mitochondrial outer membrane permeabilization (MOMP) leading to the irreversible release of intermembrane space proteins, subsequent caspase activation and apoptosis. The affinities and relative abundance of the BCL-2 family proteins dictate the predominate interactions between anti-apoptotic and pro-apoptotic BCL-2 family proteins that regulate MOMP. We highlight the core mechanisms of BCL-2 family regulation of MOMP with an emphasis on how the interactions between the BCL-2 family proteins govern cell fate. We address the critical importance of both the concentration and affinities of BCL-2 family proteins and show how differences in either can greatly change the outcome. Further, we explain the importance of using full-length BCL-2 family proteins (versus truncated versions or peptides) to parse out the core mechanisms of MOMP regulation by the BCL-2 family. Finally, we discuss how post-translational modifications and differing intracellular localizations alter the mechanisms of apoptosis regulation by BCL-2 family proteins. Successful therapeutic intervention of MOMP regulation in human disease requires an understanding of the factors that mediate the major binding interactions between BCL-2 family proteins in cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol 2010; 11: 621–632. - PubMed
-
- Goldstein JC, Waterhouse NJ, Juin P, Evan GI, Green DR. The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat Cell Biol 2000; 2: 156–162. - PubMed
-
- Rehm M, Dussmann H, Janicke RU, Tavare JM, Kogel D, Prehn JH. Single-cell fluorescence resonance energy transfer analysis demonstrates that caspase activation during apoptosis is a rapid process. Role of caspase-3. J Biol Chem 2002; 277: 24506–24514. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

